High-capacity memory, network, and part 11 compliant paperless data recorders greatly increase work efficiency while eliminating data recording errors.
With food and pharmaceutical manufacturing sites, various product quality-related numerical values related to product quality, such as temperature, flow rate, and degree of vacuum, are measured, recorded, and controlled. Traditionally, paper-based recorders have been the mainstream for recording numerical values.

Figure 1: Worker reviews data present on paperless recorder
However, there is a growing demand for paperless systems to improve production efficiency and ensure quality. Along with this, when drugs are to be marketed overseas, they must be managed in accordance with regulations established by the U.S. Food and Drug Administration (FDA). In order to save the measured values as electronic data, it was necessary to use a recorder that complies with this regulation.
Challenges
Food and pharmaceutical manufacturers have had several challenges with paper-based data recording:
- Operators run the recorders out of paper or ink, resulting in no data being captured.
- Frequent maintenance and refill of paper recorders cause downtime.
- Storage space must be allocated to secure and retain the reams of recorded data to prevent loss or tampering.
- No remote access to the data. Reviewers must physically be located at the site to view printed paper inside the recorder.
- Alarms from anomalies must be manually raised and checked on-site. Which is problematic in terms of production efficiency and quality assurance
- In clean rooms, workers must change clothes each time when checking and retrieving data output on paper. Checking multiple times a day would greatly increase work hours.
- To analyze data to identify problems, you need to manually enter the values from paper into a spreadsheet or program. Some measurements are taken at very short time intervals, resulting in a huge number of inputs.
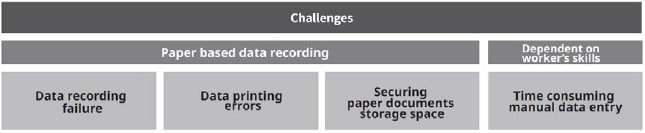
Figure 2: Paper based data recording challenges
Solution
To solve these problems, a paperless digital recorder that can store measurements as electronic data is required.
- This eliminates data loss due to paper shortages.
- Does not require large storage space.
- Enables reviewers to check data remotely over a network.
- Data can be read directly into a PC or other device, so there is no need to manually input the data.
However, you should be careful when storing electronic data of measurements used in the production of pharmaceutical products. Products manufactured in or exported to the United States must be controlled in accordance with regulations established by the Food and Drug Administration (FDA).
The FDA has established, 21 CFR Part 11, a regulation for electronic records and signatures. In order to store measured values as electronic data, a recorder must be compliant with those FDA regulations. This is to ensure that data cannot be tampered with and that electronic signatures can be recorded properly.
Key Benefits
Yokogawa’s DXAdvanced DX1000/2000 data acquisition stations address all challenges. The DX1000/2000 is a network-compatible paperless data recorder with 400 MB of internal memory and a compact flash memory card for external storage media.
- High level data security is achieved by saving data in binary file format
- Complies with Part 11 standards for electronic records through the batch and logging functions
- With an optional USB interface, data can be stored on a USB flash drive
- Network function includes Ethernet, Modbus, and other network connections. The standard implementation of Ethernet allows the use of network features such as email notifications, remote monitoring via the web, file transfer via FTP, and time synchronization via SNTP
- The network functions of the DX1000/2000 and the DAQSTANDARD software included with the DX1000/2000 make it easy to change settings from a PC. Data can also be monitored remotely using a web browser.
- Remote monitoring and e-mail notification of alarm information in the event of an anomaly have ensured quality and shortened response times. As a result, work efficiency has been greatly improved by eliminating the need for data collection and input.

Figure 3: DX1000 and DX2000
Industries
-
Food & Beverage
The food and beverage industry must produce safe, high-quality foods and beverages for consumers. In addition to quality control, the manufacturing processes include many challenges such as managing ingredients, improving efficiency and handling global environmental issues. Yokogawa leverages its decades of technological expertise to help customers build and operate the ideal factory.
-
Pharmaceutical
Under continual pressure to increase profitability, maintain government compliance, and meet emerging market opportunities, the pharmaceutical manufacturing industry faces unique challenges that require unique solutions. As one of the world’s leading industrial automation suppliers, Yokogawa is poised and prepared to deliver those solutions, creating individualized lean manufacturing techniques that deliver consistent, measurable results.
Related Products & Solutions
-
Special Solutions of OpreX Transformation
Introducing an OpreX Transformation special solution: OpreX Batch Solution
Have Questions?
Contact a Yokogawa Expert to learn how we can help you solve your challenges.