The measurement of sodium ion concentration (Na⁺) in a solution (pNa) of a process is crucial for effective process control, quality assurance, environmental management, sensor calibration, and asset protection. It allows operators and engineers to make informed decisions and maintain optimal operating conditions.
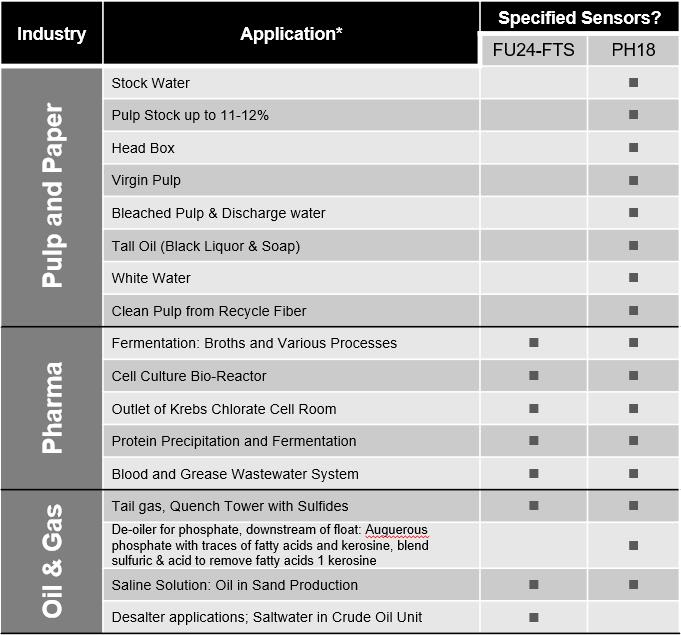
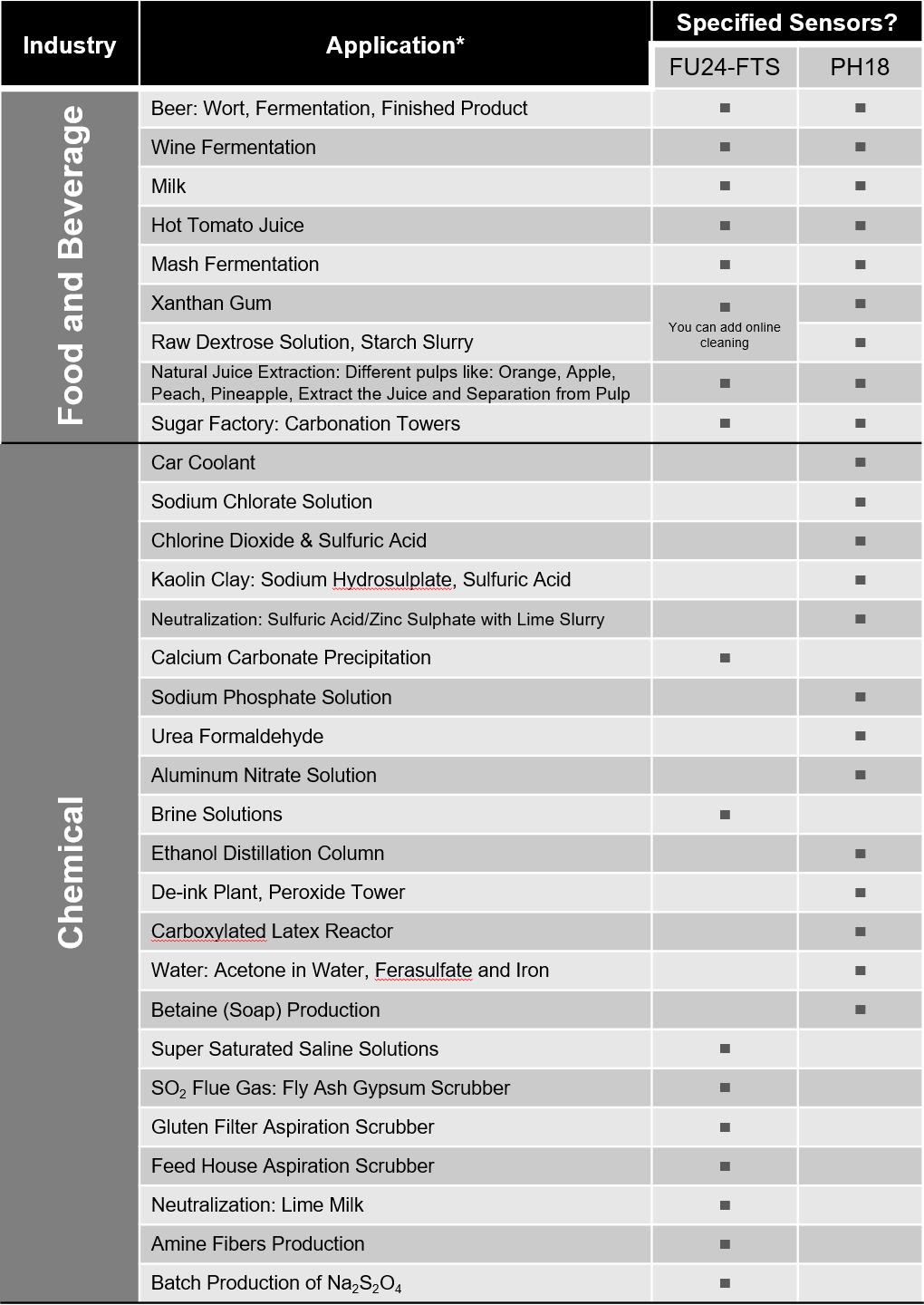
*Not every application is shown; these represent most of the installations/applications by Yokogawa.
Proper Application
When determining if the cation differential sensor can be used in a particular application there are two main requirements that must be considered:
- Is the process control pH value higher than the process pNa system + 2?
- It is very important to know the pNa value of the process because the operating range of FU24-FTS differential sensor is defined as (pNa + 2) – 14 pH. The reason is the pNa Cation reference cell is affected by changes in pH when the acid concentration is much higher than the salt concentration.
- How much does the salt concentration of the process vary; or what is the conductivity of the process at 7.00 pH?
- If the process salt concentration varies ≤ 40%, then the influence on the pH sensor will show a shift will of < 0.15 pH.
- If the salt concentration change is ≤ 25%, then the influence on the pH sensor will show a shift of only < 0.1 pH.
The concentration shift is a linear function while the pH value is a logarithmic function. This relationship is very important to understand because if the pH control needs to be maintained at ±0.1 pH, the salt concentration must not change by more than ± 25%. If it does, a new process calibration must be done to account for the salt change.
Therefore, in order to determine if the cat-ion differential sensor will work in a particular application, one of the following must be provided:
- The minimum and maximum salt concentration values throughout the process.
- The conductivity value of the process at a pH of 7.00. (The conductivity is measured at a pH of 7 to ensure the conductivity value is that of the cat-ions in the process and not the influence of the acid or base in the process.

Example 1
If the process conductivity value at 7.00 pH is 1.03 mS; then the sample would have a concentration of 500 mg/kg and a pNa of 2.08. Since the operating range of the FU24-FTS sensor is defined as pNa + 2, this would mean the sensor would function between a pH range of 4.08-14.00.
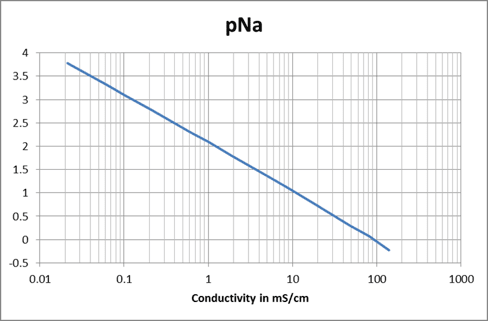
Knowing the concentration at 7.00 pH allows you to calculate the theoretically pNa of the process and the pH shift you would expect to see if the salt concentration changes. Also keep in mind that if there is a mixture of different salts, you cannot tell how much the mix will influence the reference voltage. In this situation, the best practice to measure the process.
Related Products & Solutions
-
All-in-One pH/ORP Sensor Series FU20 and FU24
The FU20 and FU24, all-in-one pH and ORP, sensors show how Yokogawa applies the motto "Simple is best" to sensor technology.
-
Differential pH/ORP Sensors
The cation differential pH and ORP sensors were designed for difficult applications where conventional sensors are ineffective. These include measurements such as brine solutions to applications as diverse as electrolysis processes and cheese manufacturing.
-
pH and ORP Sensors
- pH and ORP sensors
- Proper pH electrode/sensor selection critical for optimal measurement results
- Installation options include retractable, flow thru, immersion, and direct insertion