The disruptive technologies that form the building blocks of Pharma 4.0 are often developed by external providers of the pharma-biopharma industry. The external providers maybe legacy solution providers who have presence in other sectors such as oil and gas that have already adopted industry 4.0. Or, they maybe niche start-ups that have nimbly innovated in the area of AI-ML. A pharma company faces the challenge of incorporating one or more disruptive technologies from external providers in its journey towards Pharma 4.0.
A pharma company has to consider several aspects when evaluating a disruptive technology from an external provider. The effectiveness of the technology is the primary aspect. But even when the technology has passed the proof-of-concept stage, a pharma company is faced with questions such as – will the knowledge be transferred effectively to us, how will the technology disrupt our workflows, how do we establish systems to train our staff on the new workflows, etc. On the business front, a company has to consider questions such as – is the technology open source, how is the IP shared especially if the disruptive technology uses AI-ML, how does the business model of the provider affect our business model or even how to estimate the ROI of a technology whose returns compound over time.
A pioneer in providing Digital Twins of Bioreactors
In this article, Yokogawa Insilico Biotechnology, a pioneer in providing Digital Twins of Bioreactors will discuss its approach to these questions. Insilico will also discuss its business model and how it strives to develop a win-win model for the biopharma industry and itself.
Industry 4.0 has revolutionized the manufacturing process, through the of Internet of Things (IoT), AI and connecting different phases and components of the manufacturing process it had no doubt brought about tremendous benefits to manufacturing. Industry 4.0 approaches have greatly increased process knowledge and led to a more efficient manufacturing process which in turn reduced time and costs.
Although a lot of industries like aerospace and automotive industries have widely implemented these technologies, still the Biopharma Industry is taking its first steps in adopting Industry 4.0. Although the biopharma industry has in recent years-faced huge demands for developing new therapeutics and if Industry 4.0 was already established in Biopharma, it would have been of a great impact to reduce the high costs and time needed for producing new therapies. Biopharma companies that started to adopt Industry 4.0 are those that strive to stay ahead of others.
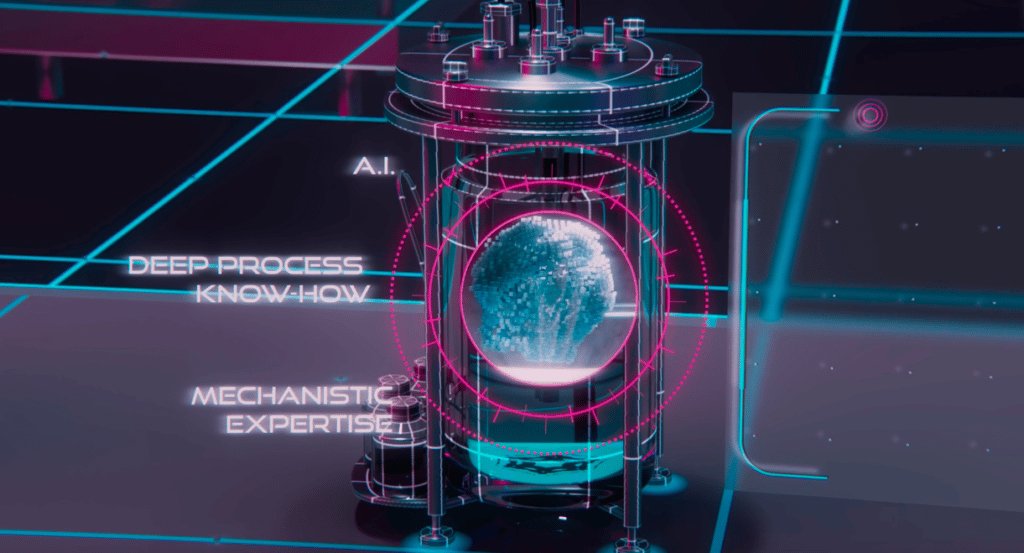
The wide implementation of Industry 4.0 in the Biopharma Industry was delayed due to several impediments on different levels. One of the main obstacles is that these innovative technologies are not developed by the Biopharma companies and thus require contracting technology providers. On one hand, this fact poses extra challenges to the Biopharma companies requiring the highest privacy policy for their data and procedures, and on the other hand, the technology providers would like to protect the Intellectual Property of their technologies. These precautions added extra procedures to regulate the collaboration between the Biopharma industry and the Industry 4.0 providers.
A long-term-investment
Moreover, if we take into consideration the highly regulated manufacturing workflow in Biopharma, we can imagine how hard it is to interrupt this workflow to adopt new technologies like the Digital Twins or to provide extra data in the initial phases to train the Digital Twin to ensure accurate predictions. In the short term, these data requirements and adopting Digital Twins within the workflow might seem costly and time-consuming, however, the decision-makers would have to see it as a long-term investment and realize the opportunities that this technology offers. This fact poses a challenge to the decision-makers to estimate the return on investment of implementing such technologies. An additional consideration would also be the time consumed to train the workforce to use these new technologies.
Besides the uncertainty of the return of investment from the Biopharma manufacturers’ side, the Business models for revenue sharing between the Biopharma companies and the technology providers are not yet well developed.