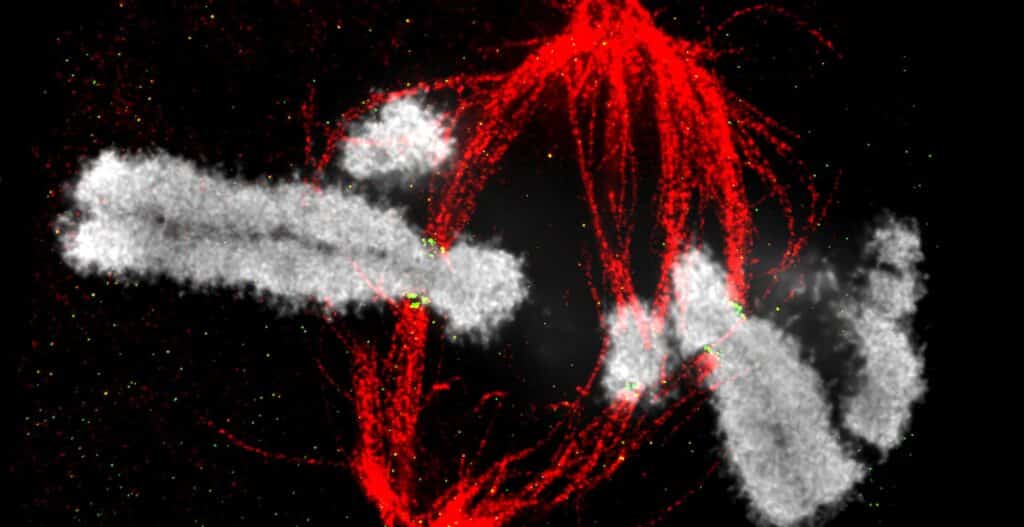
Cell division is the reproduction process by which replicated chromosomes must be equally partitioned to daughter cells. Errors in this machinery are known to be a cause of cancer and other developmental diseases. Kinetochores, which serve as the linkage between chromosomes and microtubules, play essential roles in separating sister chromatids during mitosis.

Exploring chromosome and kinetochore spatiotemporal organizations ensuring faithful cell division
Our group focuses on understanding the mechanisms behind how cells ensure the fidelity of cell division over many cell-division cycles. Cell division is an evolutionarily conserved process in which replicated chromosomes containing genetic information are equally partitioned into daughter cells. Errors in this process result in chromosomal instability (CIN). Aneuploidy, a gain or loss of chromosomes, is one of the main features of CIN and a hallmark of tumors; >90% of solid tumors and ~50% of blood tumors exhibit aneuploidy. This makes the chromosome segregation machinery one of the major targets for cancer chemotherapy.
The kinetochore, a macro-molecular protein complex at the primary constriction site of human chromosomes, orchestrates chromosome segregation by directly binding to microtubules. Around 25 different proteins contribute toward core-kinetochore architectures, and we previously quantified protein copy numbers and stoichiometry in a cell using the Yokogawa CSU spinning disk confocal microscope 1. We found that kinetochores exhibit structural deformations to control the affinity between kinetochores and microtubules using an optimized 3D fluorescence colocalization method we had developed 2,3. To understand how the kinetochore generates and transmits forces onto chromatin, we developed a FRET (fluorescence resonance energy transfer) tension biosensor to detect kinetochore tension in a cell. Using this sensor, we demonstrated that the kinetochore works as a complex force coupler, rather than a simple signal transmitter, in which multiple kinetochore proteins share the force load behind orchestrated chromosome movements 4,5. We are now exploring the mechanism underlying the spatiotemporal organization of kinetochores and chromosomes.
High spatiotemporal imaging using CSU-W1 SoRa to study the kinetochore architecture?
It is challenging to study chromosome segregation using fluorescence microscopy because it is a short-lived event, is highly dynamic, and is regulated by nanoscale structures. The size of a human kinetochore is ~200 nm and it links ~20 microtubules to chromatin. Kinetochores dynamically deform in a force-dependent manner; however, the mechanism and functions of this deformation are still unknown. We recently developed a novel quantitative and high-throughput microscopy analysis software, called 3D-Speckler (3D fluorescence speckle analyzer), which provides sizes, signal intensities, and 3D coordinates of fluorescence particles, such as kinetochores, with 1 nm accuracy6. This study also showed that the Yokogawa CSU-W1 SoRa achieves ~150 nm lateral and ~390 axial resolution without any post-image processing. Combining 3D-Speckler and CSU-W1 SoRa, we explore the mechanism of how kinetochore deformation and chromosome movements ensure the fidelity of mitosis in cancer cells.
Synergy between 12x mExM and Yokogawa CSU-W1 makes invisible structures visible
We recently developed a novel super-resolution microscopy technique, called 12x modified Expansion Microscopy (12x mExM) 7. ExM is a unique method for overcoming the diffraction limit optical barrier where biological specimens (e.g. tissues, culture cells, and organoids) are embedded into polyacrylamide-based hydrogels and expanded via hydro-osmotic expansion. Whereas most commonly used ExM techniques achieve 2~4-fold expansion, our 12x mExM achieves stable, 3D isotropic 12-fold expansion and achieves 15~20 nm lateral and ~50 nm axial resolution with the Yokogawa CSU spinning disk confocal microscope, resolutions competitive to serial block face SEM (scanning electron microscopy). Examples are shown in Figure 1 and a movie (Figure 1C). Rat kangaroo PtK2 cells, which have 14 chromosomes, were stained for kinetochores, microtubules, and chromosomes. Images were taken as 400 nm step z-stacks (equivalent to ~33 nm z-step in pre-mExM) for an entire mitotic cell. Figure 1A is a maximum projection of 5 serial sections to visualize a single chromosome (No any post-image processing applied). Figure 1B is a reconstituted 3D image with surface annotations for chromosomes and kinetochores. Although pre-mExM images were unable to distinguish individual chromosomes, 12x mExM images could identify complete sets of individual chromosomes and visualize detailed structures at the kinetochore-microtubule interface. The resolution of 12x mExM alone was sufficient to determine the karyotype based on chromosome volumes and kinetochore positions without chromosome specific FISH (Figure 1B and 1C (Movie): Different chromosomes were annotated using different colors).
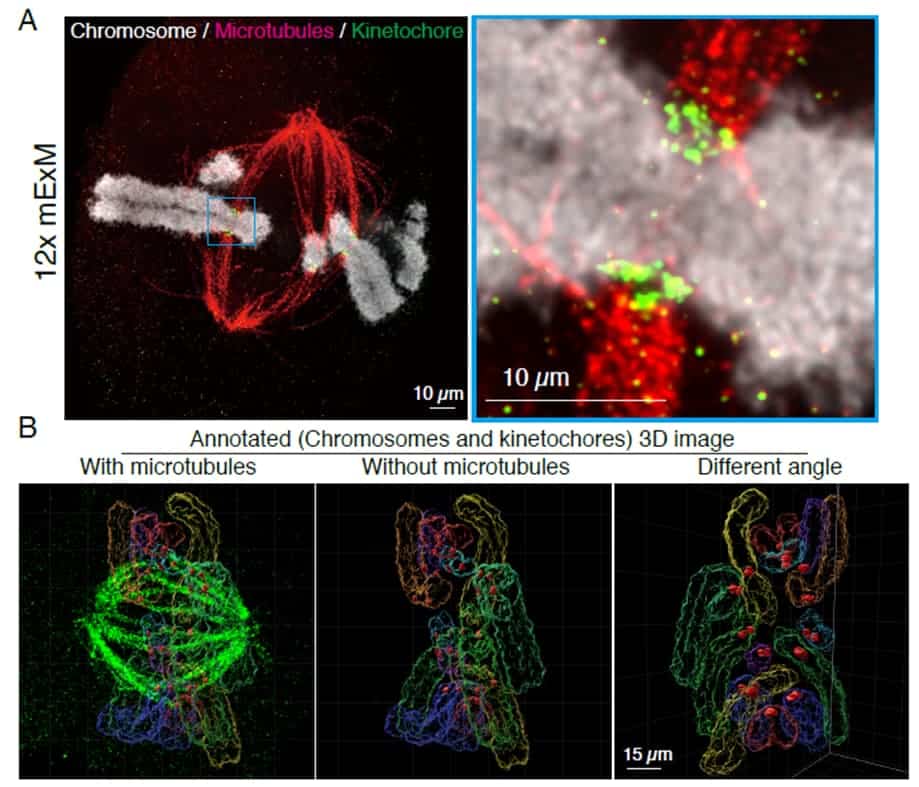
(A) An example 12x mExM image of mitotic rat kangaroo PtK cells, which have 14 chromosomes. Cells were stained by microtubules (red), kinetochores (green), and chromosomes (white). An image was taken by CSU-W1. The image was a maximum projection of 5 serial sections, but no post-image processing applied. An enlarged image is shown on the right.
(B) An example 3D reconstituted image of the expanded metaphase PtK cell. Surface of chromosomes and kinetochores are annotated.
The combination of our 12x mExM and the Yokogawa CSU-W1 (with Uniformizer) is critical for our study. Since the cell expands to 〜200 µm laterally and axially, a few hundred z-stack images with a full FOV (field of view) is required for 12x mExM 3D image reconstitutions, making the speed, illumination flatness, resolution, and minimum photo-bleaching provided by the CSU-W1 with Uniformizer necessary for our work. We are currently exploring the chromosome spatial organizations and kinetochore functions required for faithful chromosome segregation using these state-of-the-art imaging techniques.
Prospect for the future
In addition to exploring the chromosome segregation machinery, we continue to develop novel experimental and analysis methods for making invisible structures visible for understanding the underlying biology. Recently, we succeeded in visualizing a single EBV (Epstein-Barr Virus) as well as a single RNA molecule in a cell using our 12x mExM and the Yokogawa CSU confocal microscope 7. Combining 12x mExM and the CSU will be a useful in visualizing the SARS-CoV-2 virus in a cell to understand its infection mechanism. We hope our imaging technique continues to synergize with Yokogawa’s imaging equipment and contribute to not only understanding chromosome segregation, but also contributing towards progress in broad research fields.
References
- Suzuki, A., Badger, B. L. & Salmon, E. D. A quantitative description of Ndc80 complex linkage to human kinetochores. Nature communications, 6, 8161, (2015), PMCID: PMC45697.
- Suzuki, A., Long, S. K. & Salmon, E. D. An optimized method for 3D fluorescence co-localization applied to human kinetochore protein architecture. Elife, 7, (2018), PMCID: PMC5764572.
- Suzuki, A., Badger, B. L., Wan, X., DeLuca, J. G. & Salmon, E. D. The architecture of CCAN proteins creates a structural integrity to resist spindle forces and achieve proper Intrakinetochore stretch. Developmental cell, 30, 717-730, (2014), PMCID: PMC4237209.
- Suzuki, A., Badger, B. L., Haase, J., Ohashi, T., Erickson, H. P., Salmon, E. D. & Bloom, K. How the kinetochore couples microtubule force and centromere stretch to move chromosomes. Nature cell biology, 18, 382-392, (2016), PMCID: PMC4814359.
- Suzuki, A., Gupta, A., Long, S. K., Evans, R., Badger, B. L., Salmon, E. D., Biggins, S. & Bloom, K. A Kinesin-5, Cin8, Recruits Protein Phosphatase 1 to Kinetochores and Regulates Chromosome Segregation. Current biology, 28, 2697-2704 e2693, (2018), PMCID: PMC6267807.
- Loi, J., Qu, X., Suzuki., A. Semi-automated 3D fluorescence speckle analyzer (3D-Speckler) for microscope calibration and nanoscale measurement (under revision)
- Norman, R., Loi, J., Recchia, E., Rosemarie, Q., Lesko S., Patel, S., Sherer, N., Sugden, B., Takaku, M., Burkard, M.E., Suzuki., A. An optimized 12-fold modified expansion microscopy (12x mExM) (in preparation)