Introduction to my research
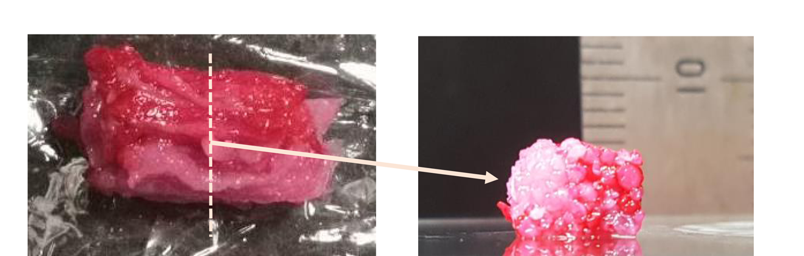
With the current interest in cultured meat, mammalian cell-based meat has mostly been unstructured. There is thus still a high demand for artificial steak-like meat. We demonstrate in vitro construction of engineered steak-like tissue assembled of three types of bovine cell fibers (muscle, fat, and vessel). Because actual meat is an aligned assembly of the fibers connected to the tendon for the actions of contraction and relaxation, tendon-gel integrated bioprinting was developed to construct tendon-like gels. In this study, a total of 72 fibers comprising 42 muscles, 28 adipose tissues, and 2 blood capillaries were constructed by tendon-gel integrated bioprinting and manually assembled to fabricate steak-like meat with a diameter of 5 mm and a length of 10 mm inspired by a meat cut. The developed tendon-gel integrated bioprinting here could be a promising technology for the fabrication of the desired types of steak-like cultured meats.
Why is it difficult to produce cultured meat?
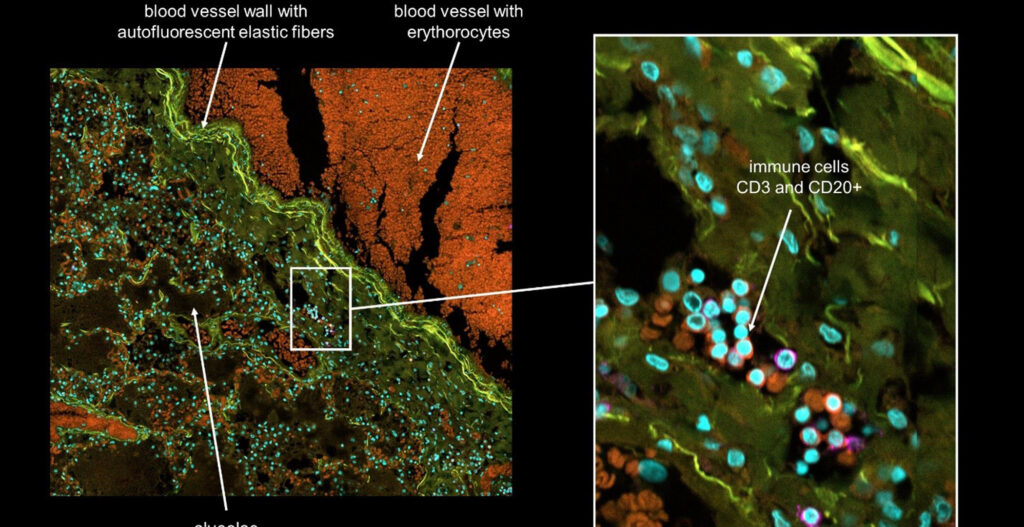
Steak meat has an aligned structure of skeletal muscle fascicles with a diameter from around 900 μm to 2.3 mm depending on age and animal parts. It is formed by assembled skeletal muscle fibers, connected to the tendon for its shrinkage and relaxation movements. The muscle fibers are covered with a basement membrane and the muscle fascicles are surrounded by fat together with blood capillaries. The component ratio and location of the muscle, adipose tissues, and blood capillaries are significantly different according to the meat type and its country of origin. For example, red meat in the rump of Japanese Wagyu has only 10.7 % adipose tissue, whereas the sirloin of the Wagyu has 47.5 %. Accordingly, the development of a methodology for assembling the three types of fibers with the desired location, ratio, and amount will be a key manufacturing technology of cultured steak.
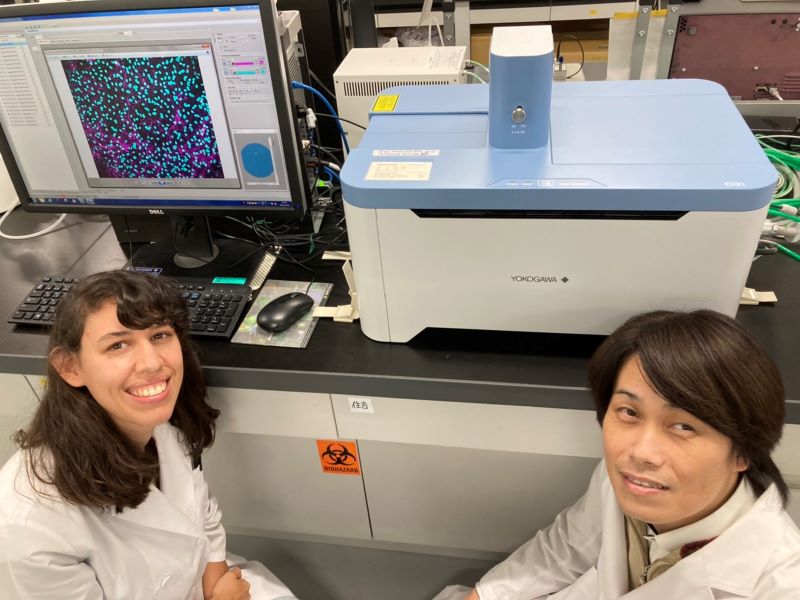
How our research did benefit from using a CellVoyager CQ1
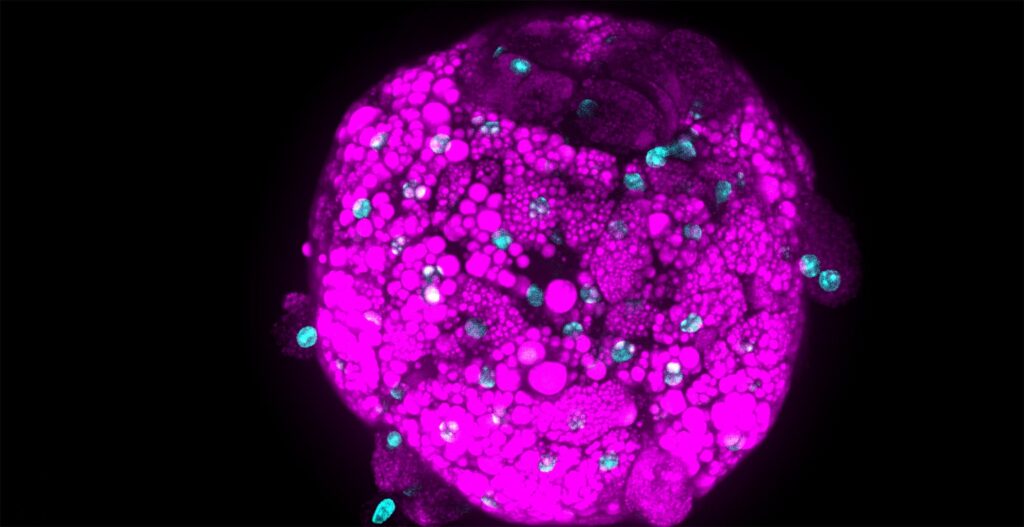
We needed to analyze multiple culture medium conditions in order to find the right differentiation medium to make bovine ADSC become adipocytes and endothelial cells. Using CQ1 allowed us to process at the same time and quite fast a large number of 3D samples seeded in either 48 or 96 well plates. This enabled us to save important time and to get reproducible and quantitative statistical data. Thanks to these analysis results we could find the right fatty acids to ensure the proper lipids storage into the bovine adiposed-derived stem cells (ADSC ) until becoming adipocytes (Figure 3) and we were able to surprisingly identify horse serum as a strong inducer of their endothelial differentiation (Figure 4).
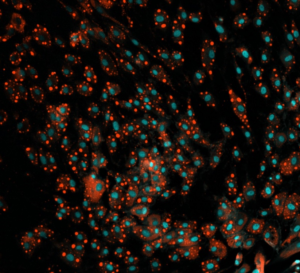
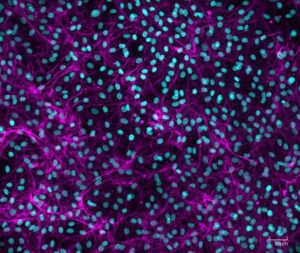
The strong benefit of the CQ1 device is its very user-friendly interface which enables to quickly prepare the imaging of the sample. It is a polyvalent device that can be used either with multiple well plates or glass-bottom dishes so it has multiple possible applications! We were impressed with how quickly the device can imagine a whole 96 well plate, even in Z-stack for 3D imaging! It allowed us to quickly process high-content imaging and analysis and efficiently move on in our research.
Prospects for the cultured meat project
The next step of this cultured meat project is to improve the cell culture medium and the cell differentiation but in an edible way to reach the production and be able to provide it soon to the population. CQ1 will help us again for sure to quickly screen multiple conditions until reaching the most suitable ones!
References
Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting
* Dong-Hee Kang, Fiona Louis et al.
* DOI: 10.1038/s41467-021-25236-9<https://doi.org/10.1038/s41467-021-25236-9>