Challenge of Elucidating Mechanism of SARS-CoV-2 Infection
Nowadays, this virus name is well known worldwide. The SARS-CoV-2 virus challenges societies and their health systems around the globe. Vaccination has already started in many countries, and there are high expectations for its effectiveness. However, many aspects of the virus infection, such as cell entry or virus propagation and the devastating effects on the patient’s health and organs are still unclear.
Our High-Content Imaging microscopes and analysis software suite can help to move further on the long and slippery road to overcome this global health crisis.
When the pandemic started last year, Yokogawa decided to collaborate with research projects worldwide with the aim to better understand SARS-CoV-2 infection. An initial blog article (see here ) describes our collaboration with the Pathology Department from the Charité in Berlin, Germany. Their retrospective research approach is aiming to identify the cause of dying by using biopsy material from COVID-19 victims. However, as the pandemic is spreading worldwide, there is a strong need to bring scientists together around the world.
COVID-19 research at the University of Helsinki
Dr. Giuseppe Balistreri is doing research at the University of Helsinki, Finland. In an international collaborative effort involving Finland, Estonia, Germany, UK, and Australia, his research group focuses on understanding how SARS-CoV-2 enters into human cells and why this virus infects so efficiently compared to other coronaviruses.
Dr. Balistreri: “Thanks to the support from Yokogawa, who provided us with an ultra-compact (i.e. suitable for BSL3 labs), high-content dual spinning disk confocal microscope – the CQ1 – we identified an important piece of the puzzle: a second virus entry factor.
We discovered that in addition to the main cellular receptor, ACE2, the new coronavirus is able to use a second cellular protein, called neuropilin-1, to gain access into human cells. When cells express both ACE2 and Neuropilin-1, the virus reaches its full infectious potential.”
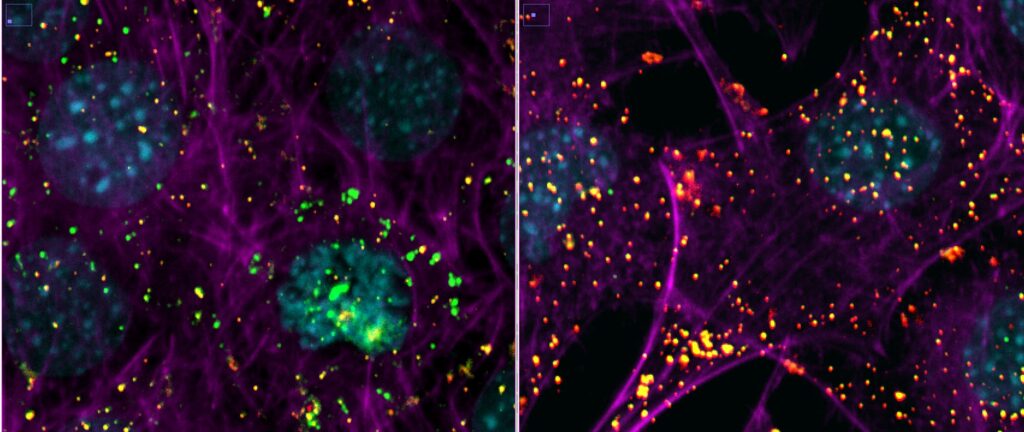
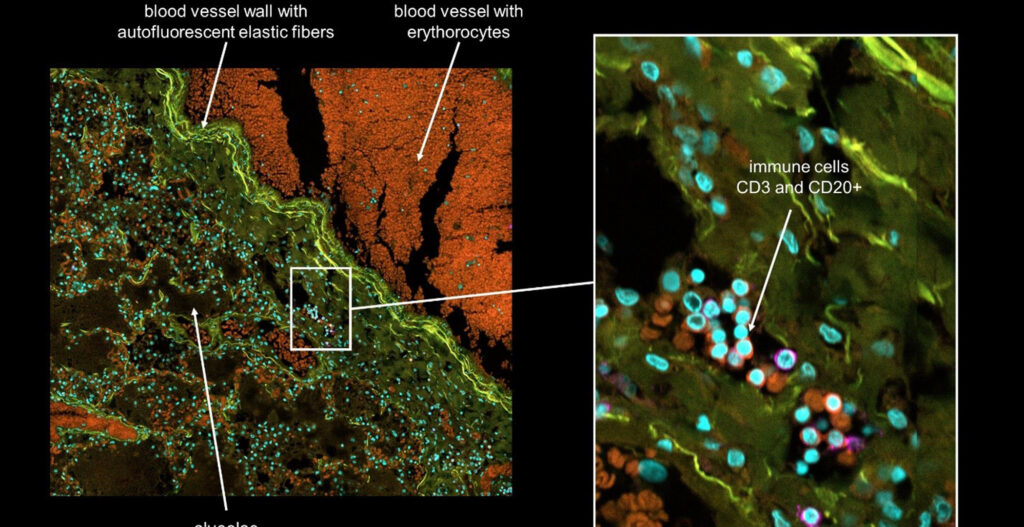
Mouse embryonic fibroblasts cells expressing the human ACE2 protein were incubated with SARS-CoV-2 for 2 hours in the presence (right image) or absence (left image) of an inhibitor of endocytosis. The infection was performed in a Biosafety Level 3 facility at the University of Helsinki.
After fixation, cells were processed for sequential immuno-fluorescence staining using antibodies against the viral surface protein spike.
The viruses that have entered the cell are visualized in green, the viruses that are at the cell surface are colored in yellow/red. The inhibitor of endocytosis prevents virus entry and almost all virions are trapped at the cell surface (right image). The nuclei of the cells are colored in cyan and the actin cytoskeleton in magenta. Images were acquired with a 40x air objective.
Images courtesy of Dr. Giuseppe Balistreri and Ravi Ojha, Department of Virology, Faculty of Medicine, University of Helsinki.
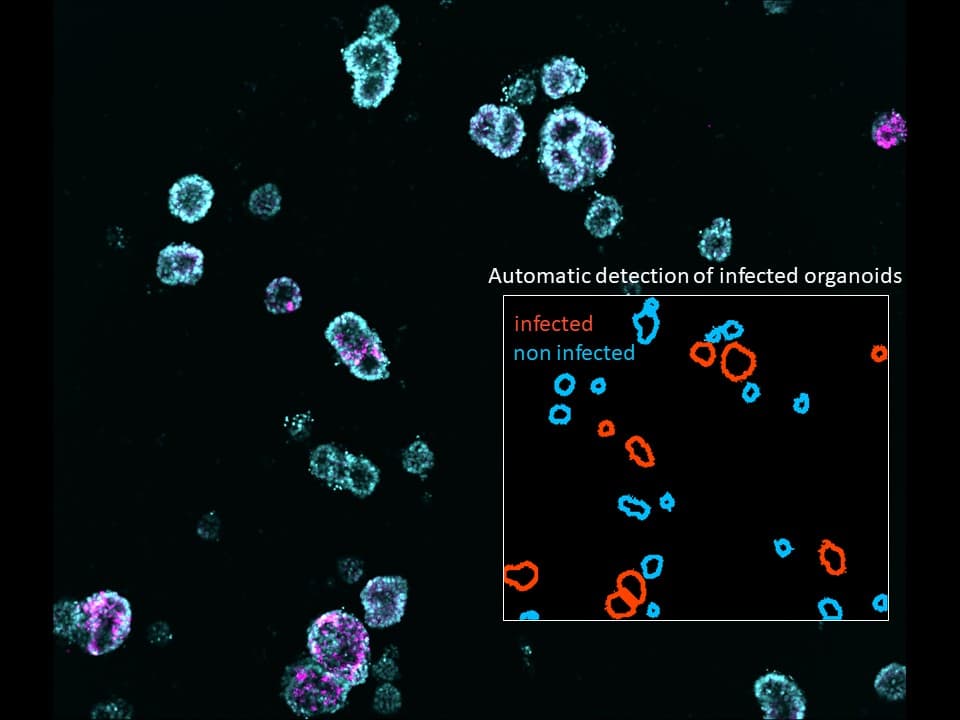
These findings, published in two papers in Science (1, 2), gave them the basis for the design of new antiviral molecules that they are currently testing in the most medically relevant model systems, including primary human airway epithelia, human organoids (Figure 2), and animal models.
[vc_cta h2=”Free On-Demand Webinar: Image-based, quantitative Approaches to study SARS-CoV-2 cell Entry and Infection” color=”peacoc” add_button=”bottom” btn_title=”Register now” btn_color=”peacoc” btn_link=”url:https%3A%2F%2Fcp.yokogawa.com%2FSARS-CoV-2-On-demand-Video.html%3Futm_source%3Dblog%26utm_medium%3Dorganic%26utm_campaign%3D0422|target:_blank” css=”.vc_custom_1649853403848{background-color: #eeee22 !important;}”]Do you want to know more about Dr. Balistreri’s research? Register for our free on-demand Webinar “Image-based, quantitative Approaches to study SARS-CoV-2 cell Entry and Infection: Looking for the Achilles’ Heel of Virus Infection”.
[/vc_cta]
Gather new findings with High-Content Screening
Dr. Balistreri: “Here the Yokogawa microscope has become the game-changer, allowing us to process a large number of 3D samples in a very short time and with high-quality. The compact size of the instrument, combined with the powerful and user-friendly software that Yokogawa has provided, allowed us to work in biosafety level 3 facilities where we can follow every step of the infection cycle. Starting from the arrival and internalization of single virions at the surface of the cell, to the formation of the so-called syncytia. A syncytia is a virus-induced phenomenon whereby cells are fused together to form a multi-nucleated gigantic virus factory.”
A Network of Scientists fighting against Sars-Cov-2
Dr. Balistreri: “In addition to providing the microscope, image analysis pipelines, and training, Yokogawa also created a network between scientists involved in COVID-19 research across the globe. Within this network, we could facilitate the exchange of knowledge, expertise, and technical experiences. I would also like to mention the excellent customer support done by Dr. Michael Schell from Cenibra, a local distributor of Yokogawa, who helped with setting up analysis routines. Thanks to this unprecedented initiative, we and our colleagues were able to accomplish in just a few months what usually would have taken years. We hope that our new antiviral strategies will become available in the near future to win the battle against this and newly emerging viral threats.”
Giuseppe Balistreri is a Principal Investigator at the University of Helsinki (Finland) since 2018 and an Honorary Adjunct Associate Professor at the University of Queensland (Australia) since 2020. For more information about his research, please visit the Balistreri lab website.
References
- Cantuti-Castelvetri, L., (2020, November 13) Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science, 370(6518), 856-860. doi: 1126/science.abd2985
- Daly, J. L. et al. (2020, November 13) Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science, Vol. 370 (6518), 861-865. doi: 10.1126/science.abd3072