Single-cell analysis enables the reconstitution of information regarding the causes of genetic changes. For this reason, it can contribute to studies on the molecular basis of cell transformation and proliferation. Techniques that require dissociation of tissue from its natural environment lead to the loss of spatial information on individual cells. But, also “State of the art single-cell techniques can measure the expression levels of thousands of genes, but they destroy the cells in the process and provide only static snapshots” (Sahand Hormoz, Harvard Medical School).
Therefore, the currently used methods for single-cell analysis as for example scRNA-seq. are providing only a limited option for discovering cell-to-cell dependent mechanisms in real-time.
Significance of SU10 for CRISPR-Cas9

Gene-editing technologies such as CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas9 (CRISPR-associated protein 9) have greatly expanded our ability to target specific cells and tissues studying their development, function, and diseases. But the ability to genetically manipulate single cells by current delivery methods including viral transduction suffers from limitations that restrict their application.
The methodology of CRISPR-Cas9 was originally found in bacteria providing acquired immunity against invading plasmids and viruses.
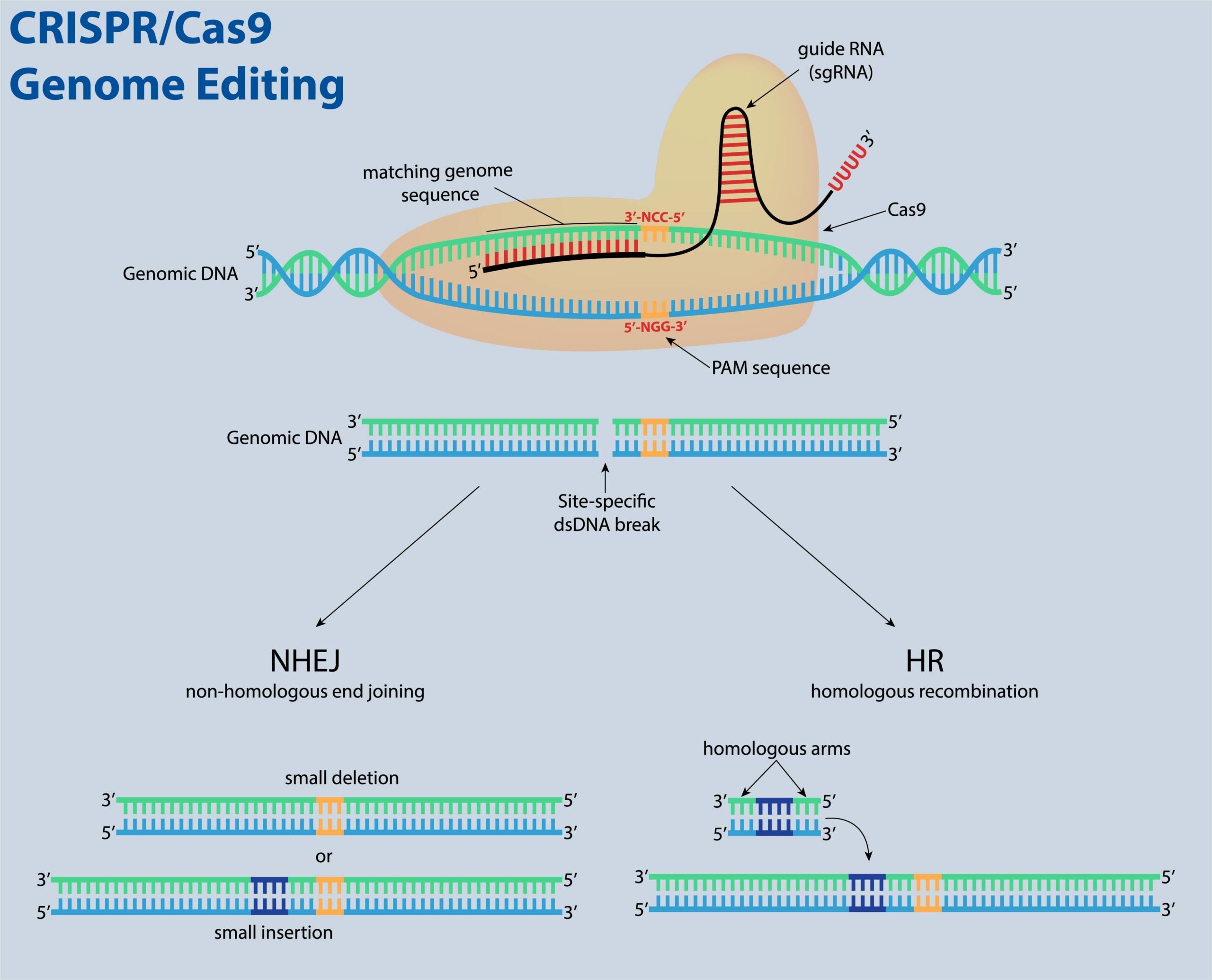
This technology allows researchers to precisely manipulate by cutting the genome either to put into or remove genetic information out of specific regions. Molecular biologically the basic principle of CRISPR-Cas9 consists of two RNA (ribonucleic acid) molecules, CRISPR- and tracrRNA together, they form the guide RNA, as well as the protein Cas9.
[vc_cta h2=”On-Demand Webinar: Nanopipette Technology – A new Tool for Single-Cell Analysis” color=”peacoc” add_button=”bottom” btn_title=”Watch Now!” btn_color=”info” btn_link=”url:https%3A%2F%2Fbit.ly%2F3gzZF5i|title:Go%20to%20Webinar|target:_blank”]Approaching sub-cellular biological problems requires the incorporation of new technologies and readouts. Due to their low invasiveness, nanotechnology-based tools hold great promise for single-cell manipulation. In this Innovation Showcase, we discuss the incorporation of electrical measurements into nanopipette technology and present results showing the rapid and reversible response of these subcellular sensors to different analytes such as antigens, ions, and carbohydrates.

[/vc_cta]
Genome editing through the CRISPR/Cas9 system
The guide RNA is customized to recruit and direct the protein Cas9 to cut the genome like scissors at the region of interest. The cell can either repair itself or a new piece of DNA can be inserted (see figure). Extraordinary therapeutic potential for treating different diseases in which the genetic cause of dysfunction is known like patients for example with hematologic diseases as the sickle-cell disease could be cured.
Comparison of different gene delivery techniques to Yokogawa’s Nano-Injector
Here we provide a unique and minimally invasive method. It enables for the first time the delivery of genetic information into single cells via a nanopipette-based approach combined with microscopy to study single-cell events in real-time, the so-called Single Cellome™ Unit SU10.
Conventional methods like Lipofection
Conventional methods for transfer of genetic material into cells like Lipofection and Electroporation introduce the genetic information only randomly into single-cells of a whole cell population. The transfection efficiencies for different cell types are varying, e.g. stem cells are often difficult to transfect. SU10 has the potential to enhance the transfection efficiency of difficult-to-transfect cells. Because the nanopipette-based technology enables researchers to introduce the genetic material directly into the nucleus, in contrast to the methods mentioned above. When using methods like Lipofection, the gene of interest needs to reach the nucleus via crossing the cell membrane and nuclear envelope.
Methods of cell injection that allow targeting of single-cells directly into the nucleus employ micropipettes. Unfortunately, these micropipettes deliver a large volume of substance that is incompatible with the size of typical cells and may harm cells. They are also difficult to operate as they are mostly non-automated or semi-automated.
Atomic Force Microscopy
In other methods, such as atomic force microscopy (AFM), hollow cantilevers were constructed, but these are also limited by lack of control of injection volumes (Ref2).
Virus Stamping Method
Another method for targeting single cells in vitro and in vivo is the so-called virus stamping method which is based on the targeted transduction of cells with adeno-associated viruses (AAVs).
Virus stamping can face distinct problems which include the need for magnets customized to fit the spatial limitations of the experimental setup and to generate sufficiently high magnetic fields (~ 120 mT).
Potential disadvantages are:
- High magnetic fields may interfere with the experimental setup
- Depth at which cells are targeted is limited by the feasibility of visually guiding the pipette to a target cell
- Slow system rate of ~ 3 cells in ~ 15 min (Ref 3).
SU10 takes it all
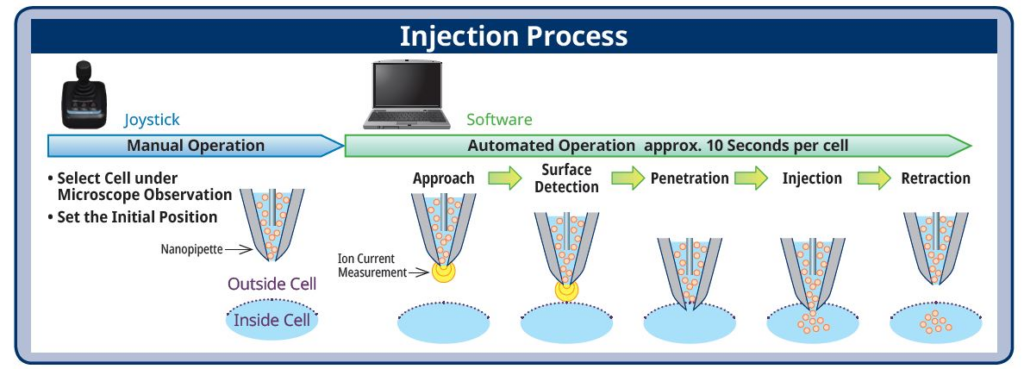
From my active scientist career, I remember that transfection was one of the most difficult methods to master. The SU10 has a success rate of approximately 95 percent which is extremely high. The risk of damaging a cell with this method is very low. Our technology is a fully automated system based on ion current measurement via detection of the nanopipette tip. It is capable of injecting one cell every 40 seconds (see figure below).
The SU10 is both fast, efficient and saves precious time because of its fully automated system. It enables you to focus on the things that you are passionate about – conducting scientific research.
Do you want to know more about the SU10? Could you imagine other application areas for the use of our product? Discuss with me in the comments or feel free to contact us (lifeinnovation@de.yokogawa.com).
[vc_separator color=”blue”]
Sources
(1) Rodríguez-Rodríguez DR, Ramírez-Solís R, Garza-Elizondo MA, Garza-Rodríguez ML, Barrera-Saldaña HA. Genome editing: A perspective on the application of CRISPR/Cas9 to study human diseases (Review). Int J Mol Med. 2019;43(4):1559-1574. doi:10.3892/ijmm.2019.4112
(2) Gonca Bulbul, Gepoliano Chaves ID, Joseph Olivier, Rifat Emrah Ozel and Nader Pourmand. Nanopipettes as Monitoring Probes for the Single Living Cell: State of the Art and Future Directions in Molecular Biology. Cells 2018, 7, 55; doi:10.3390/cells7060055
(3) Schubert R, Herzog S, Trenholm S, Roska B, Müller. Magnetically guided virus stamping for the targeted infection of single cells or groups of cells. DJ. Nat Protoc. 2019 Nov;14(11):3205-3219. doi: 10.1038/s41596-019-0221-z. Epub 2019 Oct 18. PMID: 31628446