The desire for customization and flexibility has seldom been as strong as it is today. Among other things, it’s making markets even more dynamic. Companies in the chemical and pharmaceutical industries are consequently striving to keep production as flexible as possible – from end to end. The outcome: more and more new and highly specific products in smaller volumes as well as frequent changes in production schedules. Yet in spite of this, at the end of the day, the quality must invariably be up to scratch – while helping the manufacturer to make a profit, of course. Does that sound like squaring the circle to you?
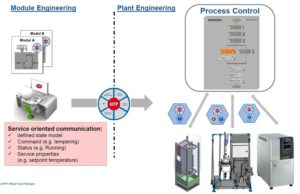
In order to respond not just flexibly but also fast, manufacturing processes must first and foremost be made less complex. The best way to achieve that is with a modular plant. Modularization encompasses everything from the initial design through the process technology to the automation system. From the engineering to automated operation, all of this can only be realized if standards exist which can be utilized by everyone. But is that even possible?
Following various demonstrators and tests, the first commercial lighthouse projects are meanwhile available, in which modules are automatically integrated into the automation system using MTP. And that’s only the beginning. Sooner or later, modules won’t simply be automatically integrated into the automation system; they’ll also communicate with one another directly and the automation system will merely be required to orchestrate them.
 Orchestrating flexibility
Orchestrating flexibility
If we take a look at the chronology of production, there are basically two types of process, namely continuous and batch. Continuous processes run without interruption. Their subprocesses are usually linear. Continuous processes are the solution of choice for processing large amounts with few product changes.
In batch processes, on the other hand, the material or substances are added at different times and in small amounts; the subprocesses are nonlinear (batch control).
Basic motives for modularization
As I mentioned earlier, there are three basic motives for modularizing a plant (and with it the automation system):
- New and highly specific products,
- Smaller volumes,
- Frequent changes in production schedules.
The easiest strategy for operators is to use automated standalone modules, which can be purchased turnkey and swapped rapidly. In the pharmaceutical industry, the “ballroom concept” refers to manufacturing areas featuring interchangeable process modules, each with their own automation system. The lighthouse projects have shown that quick and easy integration into the automation system must be feasible to the same degree as orchestrating individual process modules.
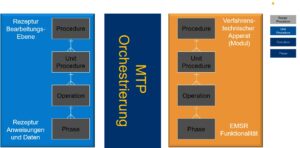
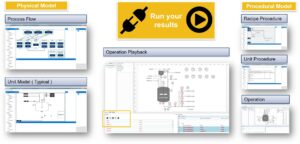
How do you handle this?
Will you dispense with a monolithic control system in the future and use only the functionality for orchestrating individual process modules in the automation system? Just how great is the demand for flexible production and modularization?
Synaptic Business Automation as the guiding theme at ACHEMA 2018
Artificial Intelligence: Far more than Terminator, K.I.T.T. or R2-D2

 Orchestrating flexibility
Orchestrating flexibility

