Consider high-fidelity online motor fuel characterization
Motor fuels are complex products. Various diesel fuel and gasoline grades are prepared from a large range of individual blending components whose individual properties may be extremely variable over time. Each day, refiners must determine the blend recipe based on the properties, value and availability of those components, as well as the target specifications for the product. Once that is done, the operational challenge is to verify that the blended product delivered to the pipeline actually meets those contractually defined specifications. Variation in catalyst and unit operations, unrelenting cost pressures and an ever-wider range of crude oil feedstocks compound the challenge. No matter how daunting these factors are, it still remains the refiner's job to manage all elements so that they are transparent to the pipeline operator.
Meeting specifications can mean either that a property value must not fall below a defined minimum or be greater than a do-not-exceed limit. An obvious example is octane in gasoline. Regulations are designed to ensure that the octane value of gasoline sold as 91 octane is, in fact, not less than 91. Yet, the pipeline operator and the consumer filling up the gas station are not concerned if the refiner delivers 92 octane instead. But, for the refiner, such property giveaway can be costly.
Do-not-exceed specifications include sulfur, Reid vapor pressure (Rvp) and benzene, for which government regulators define maximum values. Similarly, cold properties in diesel - such as cloud point, pour point and cold-filter plugging point - have maximum that vary seasonally. In general, the greater the excursion from specification limits into "safe" territory parts per million (ppm), the greater the potential profit loss for the refiner.
Complex Product
Gasoline is more complex than diesel in that it typically has a larger range of potential blending components (up to 10). While the individual components may contain 100 or even hundreds of distinct chemical compounds, the blended product may have over 1,000. The objective in blending is not to control the individual components, although toluene may be added to increase octane while butane both enhances octane and improves starting in cold weather. Rather, final blend properties are dialed in during the process by controlling the ratios of the blending components, each of which has its own unique set of properties. The fact that properties oten interact in oposing ways further aggravates the difficulties of blending. For example, adding butane boosts octane, but can raise Rvp beyond acceptable limits.
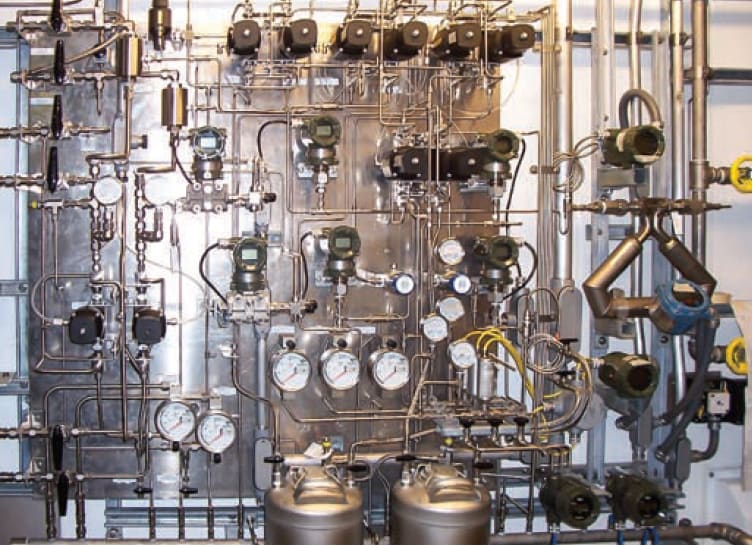
Online analysis of properties in the blended product is critical for meeting specifications and minimizing property give-away. Refiners without online property analyzers must "blend to tank" and then sample the blended batch before releasing it to the pipeline. Online analyzers do not necessarily eliminate this practice. However, real-time monitoring and feedback control to the blending optimization program help ensure that no rework (batch adjustment) will be required before release to the pipeline. Online property measurements can help minimize errors in manual sampling and testing. Also, the fact that blending control is based on hundreds of measurements (FIG. 1) instead of a few samples taken to the refinery labratory leverages the statistical benefits.
FIG. 1. Blending control is based upon hundreds of measurements.
Motor Fuel Properties
Listed here are properties that are among the many that must be certified to specifications governing custody transfer via pipelines:
- Research octane number (RON), motor octane number (MON) or pump octane, which may be calculated as an average of the two (gasoline)
- Rvp (gasoline)
- Benzene (gasoline)
- Sulfur (gasoline and diesel)
- Distillation yield points (gasoline and diesel)
- Pour point (diesel)
- Cloud point (diesel)
- Cold-filter plugging point (diesel)
- Flash point (diesel)
- Polycyclic aromatic hydrocarbon (PAH) compounds, also known as polynuclear aromatics (PNAs) for diesel
Given the immense number of properties that must be controlled during blending, a control strategy that uses online analyzers normally depends on having one analyzer for each property or group of properties. Thus, a gas chromatograph can generate values for multiple distillation points, but most other properties require a dedicated analyzer. The analyzer for octane is actually a high-tech engine, called a "knock engine." These engines are very expensive and labor intensive.
The high cost to install, operate and maintain online analyzers has led to the quest for a less complicated, more comprehensive approach. What refiners want is the ability to predict final product characteristics based on the simplest and least expensive analysis methods. For over 20 years, refiners have sought relief for this problem by predicting motor fuel properties based on inferential spectrometry. The earliest and most common example is multivariate near-infrared (NIR) spectroscopy, with Fourier transform infrared (FTIR) and Fourier transform near infrared (FTNIR) being important variants.
All of these technologies infer properties via the advanced multivariate statistical modeling discipline known as chemometrics. Proponents of a particular spectroscopy technology argue that theirs has advantages over the others. And while some do excel for very specific, technical reasons (which are beyond the scope of this discussion), none are completely satisfactory. More recently, users have considered Raman spectroscopy and nuclear magnetic resonance (NMR) technologies.
What is the problem?
No single method has emerged as a fool-proof, always reliable, one-size-fits-all approach. Though inferential spectrometry has well-established capabilities, users hoping for a perfect solution fine, instead, that ongoing fidelity demands ongoing modeling. Further, all properties apparently do not lend equally well to this inferential approach.
In an effort to make refiners comfortable with their particular type of NIR analyzer, Raman analyzer or NMR analyzer, vendors refer to the sample spectra they measure as "fingerprints." They further explain, that because each compound's spectrum is unique, their combination in various concentrations will produce a spectral fingerprint that is also unique. The job of chemometrics, then, is to correlate a mixture's fingerprint with its properties. But, as anyone reading a detective thriller knows, this analogy quickly falls apart at two levels. Frist, spectroscopy adds another dimension, in that it is not just about pattern recognition, but also is quantitative. Extending the analogy, it is like trying to have a crime-scene investigator examine a fingerprint left on a glass and using it to determine how hard a finger was pressed against the surface. Second, the fingerprints of two different gasoline blends may be quite similar (or even identical), given the large number of ways that a refiner can blend products to meet quality specifications.
Thus, the expectation is that inferential spectrometry will work for the numerous recipes that combine blending components with a variable property makeup. With the average concentration of compounds in gasoline being 100%/1,000 = 0.1%, consequently, the requirements for developing and updating chemometric models do not square with the expectations for prediction fidelity.
At issue is the fact that all compounds in a blend give responses across the same wavelength or frequency scale. What differentiates them is that their signal strength at each wavelength is not the same. But all those individual fingerprints are effectively superimposed on each other in the final spectrum of the blended product.
A musical analogy
Compare the process to recording a symphony orchestra. No matter how many instruments there are, what gets recorded via a microphone becomes a single waveform. If you examine that waveform on a computer, you can identify an instrument playing a note at 440 Hz, but you may not be able to tell if that instrument is a clarinet, violine, trumpet, something else or all of the above. If you listen to one instrument at a time, you might be able to identify what you are hearing from the waveform. However, with more instruments, it will become increasingly difficult or even impossible to distinguish among the various components that together produce the music.
Such is the case with analyzing motor fuesl: you are looking for small but significant differences in the spectrum. Many of the compounds are quite alike and have similar NIR expressions, but recognizing these small but distinct differences is the key to properly quantifying motor fuel characteristics.
Can one spectrum define the chemistry?
There is no question that the spectrum is determined by the chemical composition, and that all the individual components combine to create the final result. Given all those variables, it is no surprise that developing and keeping high-fidelity models in tune is difficult. The fact that inferential spectrometry works as well as it does attests to the technology, the high information content in the spectra, and the power of chemometric modeling.
The possibility exists that two measurably different spectra from two different blends will have practically identical properties (FIG. 2). Moreover, two spectra that are practicaly indistinguishable may have measurably different property values. In mathematical terms, a chemometric model under-determines the chemistry of the sample. It also means that properties of interest do not express themselves uniquely in a given sample spectrum. In terms of information science, the degrees of freedom in the chemistry of a calibration sample set used to make chemometric models exceed the degrees of freedom in the spectra for those samples.
Certainly, correlations exist between the properties and the information in the spectral data, but the correspondence between the chemistry and the spectral data is incomplete. So, the information from any of the measurement methods does not uniquely and completely describe the chemistry of the mix.
FIG. 2. Overlaid spectra for a number of different gasoilne blends at a single refinery. Although the overall shapes of the spectra are very similar, they differ with respect to the relative intensities of absorbance values from one end to the other.
Ranking the technology
When considering the variety of online analyzer technologies on the market, the strengths and weaknesses of each product should be thoroughly parsed. Remember that obtaining a good result depends on getting solid reference data from the lab, which then becomes the data used in making the model. This means that the modeling sample set must be representative of the full range of blend recipes.
The crux of the debate is determining what aspect has more shortcomings, the spectrometer or the modeling? The modeling algorithms are the key, since they will allow for chemometric success. However, sometimes refiners need more than the technology can deliver, and vendors do not like to admit that their product is not up to the task.
The solution to this quandary may lie with selecting the best analyzer technology for the application, and then using the data generated by the analyzers in a systematic, technically responsible fashion. NIR has been the default approach for many years for motor fuel analysis. Most users accepted it because, until about a decade ago, there was little in the way of alternatives. But the difficulty in obtaining high-fidelity property predictions has allowed other spectroscopic technology vendors to suggest that the information content in NIR spectra is insufficient.
Vendors of Raman and NMR spectrometers for online motor fuel applications have been gaining attention in the market, due to the novelty of these technologies and the disparity between the promise and performance of NIR. Chemists have long relied upon NMR, mid-IR and Raman instruments to elucidate the chemistry and structure of compounds that are nominally pure. This is because the spectral responses are discrete and lend to interpretation unaided by chemometrics. Certainly, the information they generate is more accessible, but the point is that, generally, the value of the methods is secured without chemometrics. By contrast, motor fuel property prediction is a statistical, population-based enterprise: it's a different game, and different rules apply. In practice, when working with motor fuels, Raman spectrocopy and NMR have a track record similar to NIR when it comes to predicting fuel characteristics. Indeed, claims that superior information content is the key to successful inferential spectrometry ring hollow when it comes to mixtures containing hundreds or thousands of components. On that basis, an informed spectroscopist will reason that the best performance available from any molecular spectroscopy technique cannot exceed that of the best FTNIR spectrometer.
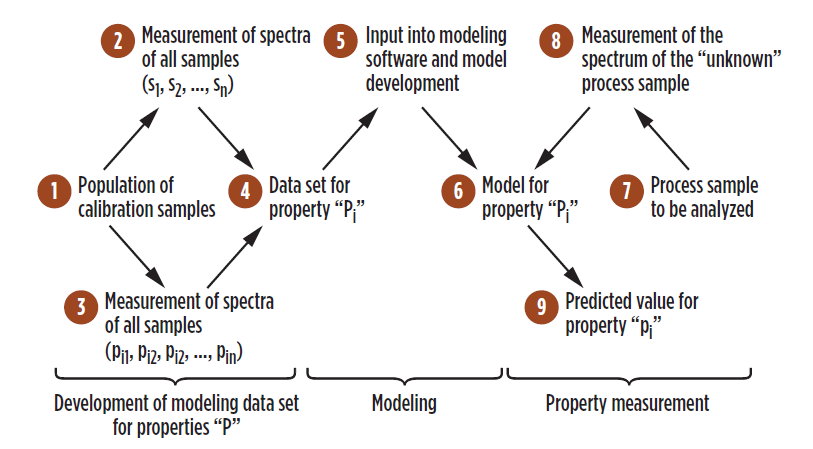
FIG. 3. Overview and implementation of inferential spectrometry models.
Solution
Is the situation hopeless? No, but the answer will not be found in looking for new technology. Instead, the best solution is to be more vigilant about maintaining and applying spectral data sets used to create property models.
Refiners seeking to apply online inferential spectrometry for motor fuels analysis have high expectations driven by their need to meet contractual obligations and maximize profits. But the noted gap between those needs and the performance delivered by inferential NIR spectrometry has caused much doubt concerning the latter, with the first assumption being that there must be something wrong with the technology.
The simple fact is that there is no magic. These technologies work, but they must be applied with discipline:
- Keep spectral data set models up to date
- Be fanatical about reference value accuracy from the lab
- Examine and test the model assumptions regularly
- Be sensible about data evaluation and realize its limitations
More specifically, inferential spectrometry for motor fuel property prediction depends on attention to elements depicted in FIG. 3. These elements include:
- Proper development and selection calibration samples used in modeling
- Transfer those samples to the laboratory in a way that preserves sample integrity, followed by properties analysis
- Model development consistent wtih best practices
- Initial and ongoing inferential prediction validation by statistical comparison
- Constant property model changes via continued modeling data set updates
Motor fuel analysis requires refiners to take control of the situation. Those involved should possess a realistic understanding of what is and is not possible, and have a firm grasp on what information can be extracted dependably. This information must then be used in an intelligent manner to continuously update the inferential analysis, a process that requires experience, engineering expertise and refined judgment.
Have Questions?
Contact a Yokogawa Expert to learn how we can help you solve your challenges.