Originally published in Pharma Manufacturing Magazine - Quality
Manufacturers that are digitalizing lab information management can improve quality and streamline operations.
As pharmaceutical manufacturers pursue quality enhancements, management is realizing many opportunities for improvement in laboratory information management and workflow processes.
Traditional methodologies are error-prone and can complicate compliance. As quality improvement teams turn to digital methodologies, they most commonly are challenged with:
- Improving the efficiency of data collection and analysis
- Improving the efficiency of quality control management
- Improving overall business efficiency
- Overcoming problems with human errors and document management
To overcome these challenges and enable improvements in laboratory data management and workflow processes, many pharma manufacturers are turning to digital laboratory information management solutions (LIMS).
THE PROBLEM WITH PRE-DIGITAL METHODS
Using traditional methodologies in which large quantities of data are transcribed, technicians find the raw data transcription to be subject to errors. Even the checking process introduces the risk of errors. Review processes often are cumbersome. It takes considerable time to check dilution calculations, blank calculations, and reported values.
Analyzing and tracing historical data, particularly when test records are maintained as paper documents, also incur considerable time. There are inefficiencies in report generation. Sometimes, there is a lack of space for storing test records as paper documents.
Older methodologies also make compliance with U.S. Food and Drug Administration (FDA) 21 CFR Part 11 and other regulations more difficult. Such processes are prone to inconsistencies in recordkeeping, which could complicate audits.

Figure1 - The industry is digitizing the pharmaceutical lab to improve quality.
‘Pre-digital’ methods further introduce difficulties when personnel outside the lab need to check on testing progress. Internally, the manufacturing and shipping departments most often need access to this information. It also takes considerable time to prepare high-quality information to respond to the requests of external parties such as customers.
DIGITALIZING THE LAB
Manufacturers that are digitalizing laboratory information management and workflow processes can realize numerous quality benefits (Figure 1). It is desirable to unify data management including the management of requests to external analytical laboratories. In a transformed operation, the unified information can be made available throughout the enterprise and the supply chain — and provided in formats that are best suited to all individuals requiring access.
In addition, unified management of analysis data and analysis workflows meets customers’ requirements for enhanced quality management and enables them to respond to recalls and complaints quickly.
To enable improvements in data management and workflow processes, manufacturers are turning to digital LIMS. The digital solutions support analytical and quality management operations and allow end users to establish test workflows to standardize and optimize laboratory operations.
Ultimately, the LIMS enhances quality by minimizing human error, improving laboratory information management workflows, increasing quality control reliability, and ensuring compliance with such regulations as FDA 21 CFR Part 11.
Tight integration of the LIMS with enterprise resource planning (ERP) platforms and manufacturing execution systems (MES) of measurement results from analyzers eliminates the need for raw data transcription. Dilution, blank, other calculations, and rounding of reported values are automated.
Automated lot-by-lot recording of test information such as the equipment in use and reagent or technician name facilitates traceability and data analysis. The LIMS manages the progress of each lot and enables the test progress to be viewed graphically from the manufacturing and shipping terminals.
It provides functions such as operational history, identification records of analyzers used for measurements and technician identifications, which are compliant with FDA nonconformities in the industry. The unified guidelines provide best practices that are intended to avoid nonconformities and regulatory agency sanctions.

Figure 2 - ALCOA+ Principles. These guidelines help to ensure the reliability of data requested by regulators in Europe, the U.K., and the U.S.
The FDA has issued five data integrity characteristics that are based on the accurate, complete, and consistent recording and management of data or information, either on paper or electronically. In the “ALCOA” concept, the five data integrity characteristics are attributable, legible, contemporaneous, original, and accurate. Figure 2 contains the definitions.
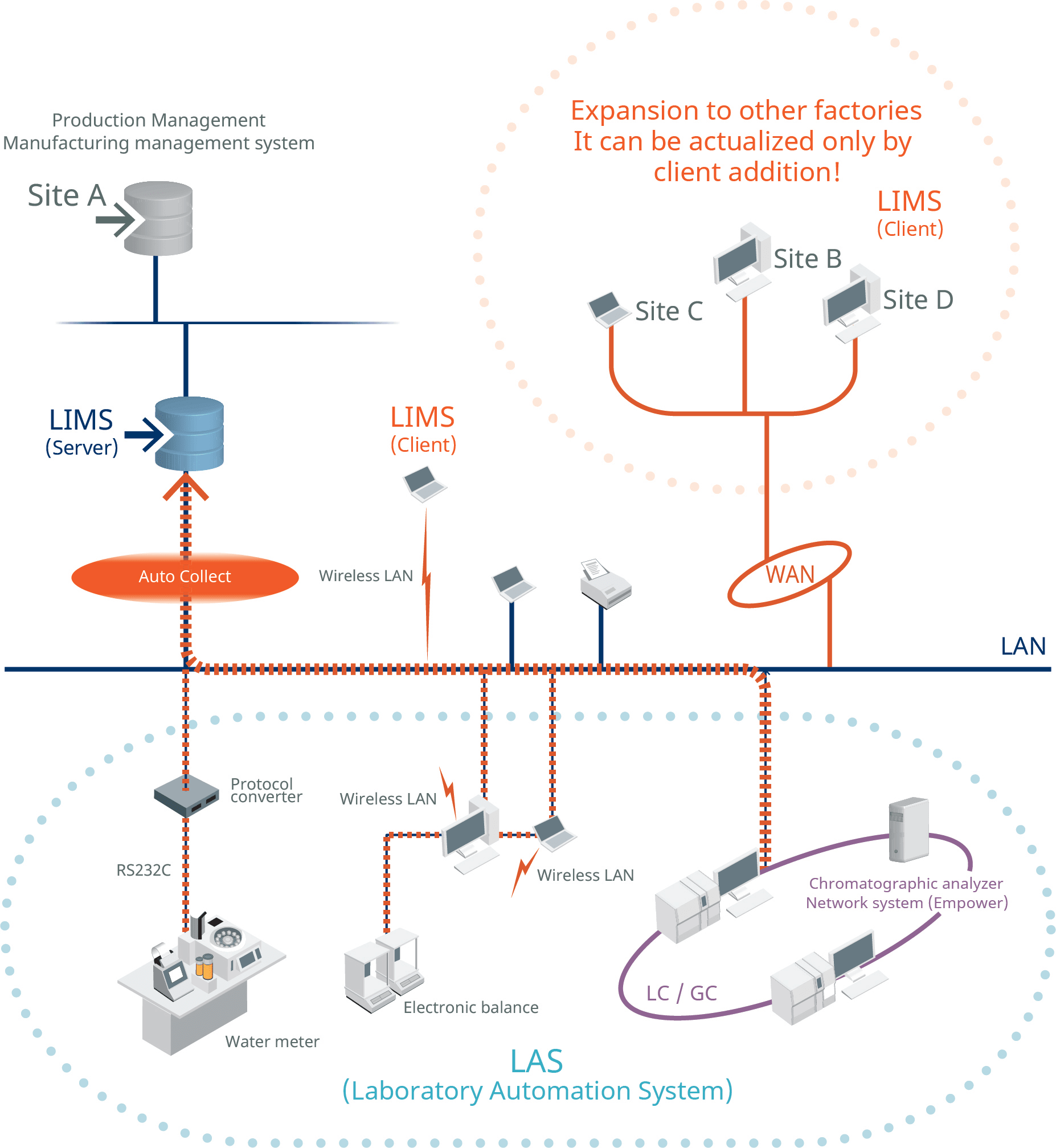
FIGURE 3 This example shows how a laboratory information management solution (LIMS) supports data sharing with other systems via a corporate network.
Further to the ALCOA concept, the PIC/S agency in Switzerland uses “ALCOA+,” which has added four more characteristics for data to be considered intact: complete, consistent, enduring and available. These characteristics also are defined in Figure 2.
In an example, among the guidelines is a criterion that the data recorded from analyzers in a lab needs to be precise upto the last decimal digit. Typically, a LIMS includes a feature, which enables technicians to achieve the quantifiable benefits of automatic input for experimental data. This provides for a considerably reduced workload for double-checking records and reports. Users have found barcodes very effective and have helped to reduce workloads in reagent and analyzer management with increased efficiency of data traceability.
STREAMLINING ANALYSIS AND QUALITY OPERATIONS
In addition to meeting data integrity, best practices are the need for quality activities in compliance with regulations such as Current Good Manufacturing Practice (CGMP). The agency “ensures the quality of drug products by carefully monitoring drug manufacturers’ compliance with its (CGMP) regulations. The CGMP regulations for drugs contain minimum requirements for the methods, facilities, and controls used in the manufacturing, processing, and packing of a drug product. The regulations make sure that a product is safe for use, and that it has the ingredients and strength it claims to have.
The approval process for new and generic drug marketing applications includes a review of the manufacturer’s compliance with the CGMPs. FDA assessors and investigators determine whether the firm has the necessary facilities, equipment, and ability to manufacture the drug it intends to market.
In the industry, there is a growing need for high-mix/low-volume production, and conventional paper-based data management often can be overly complicated. A LIMS addresses the regulations by establishing a workflow for quality control to improve efficiency, save labor, and centrally manage test data.
An LIMS also provides total support for the standard workflow of quality control, from receiving test requests and issuing instructions to the approval of test results and factory release evaluation (Figure 3). It also covers all aspects of quality control, including automated data collection from analyzers, stability test plans, and management of reagents and analytical instruments.
By linking with existing MES and ERP systems, it is possible to visualize the entire enterprise, which significantly impacts improvements at all levels from corporate management to the plant floor (Figure 4)
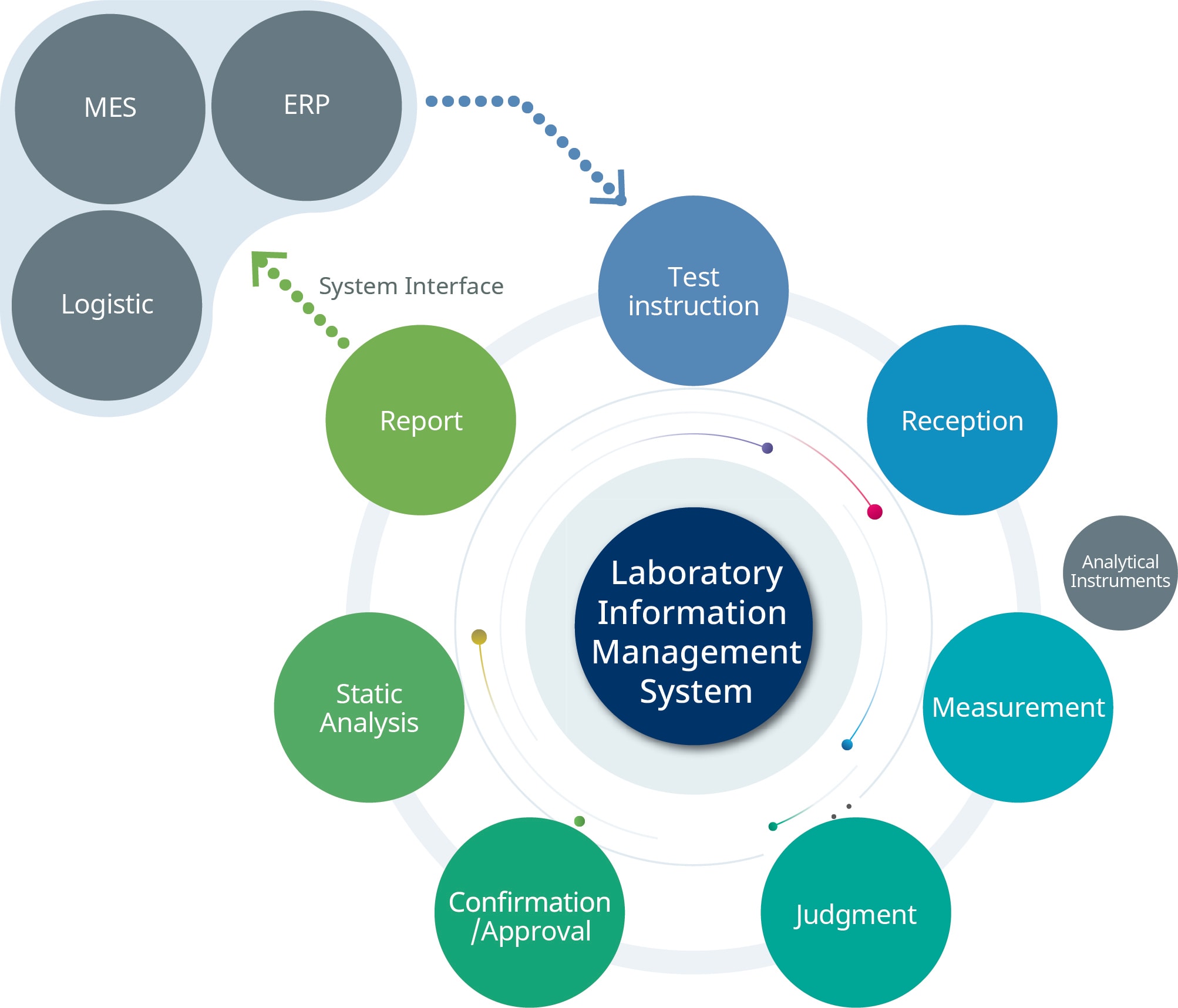
A LIMS streamlines operations by establishing test workflows and provides 21 CFR Part 11-compliant electronic signatures. It also enables centralized control of test data through online connection with inspection and analytical instruments and allows visualization of internal operations in the plant through linkage with other systems.
BENEFITS OF DIGITAL LIMS
To enable quality improvements in pharma operations from the laboratory through the enterprise, a digital LIMS offers numerous benefits.
- Standardization of quality control operations: Users can establish standard workflows to improve the quality and efficiency of analytical operations.
- Workflow reductions through lab automation: The platform allows technicians to connect online with a variety of analytical instruments and collect analytical data automatically. This has a significant labor-saving impact on data input and prevents transcription errors and data falsification.
- Regulatory compliance through security and traceability features: Operators achieve secure system operations by granting appropriate permissions to users through user ID and password at login and authorization. Also, as a traceability function, users can check the history of sample acceptance, approval of results and release evaluation.
- Paperless operations and improved search speed: Online input of test data eliminates hardcopy. Test data can be approved and released via an electronic signature. In addition, a database that includes test data and approval data allows searching at a much higher speed than paper-based data management would allow. Quickly comparing and analyzing past data allows management decisions on test data in real-time.
- Accommodation of a variety of forms: The platform allows users to freely create form layouts. This can significantly reduce the engineering costs required for form creation.
- Stability test plan preparation: A stability test can be planned to evaluate the aging deterioration of drugs. Automated test requests are sent in accordance with the plan. This can prevent missed testing time points and analytical method errors.
- Concurrent validation and retrospective validation improve data reliability: The past and present test data can be referenced by the test unit. Users also can set permissions for editing test data and viewing the audit trail of any alteration.
LIMS provides a broad variety of both intangible and quantifiable benefits. Among the former are the elimination of human error in data entry, transfer and calculations and increased efficiency in analytical and experimental tasks via standardization. High data
traceability improves customer satisfaction, and improvements in product quality and compliance operations lead to an increased reliability in company-wide quality management.
Quantifiable benefits include the automatic input of experimental data; a reduced workload for double-checking; and a reduced workload for instructions, records, and reports. Barcode scanning reduces the workload in reagent and analyzer management. The LIMS further enables integration with many other business systems and substantially increases efficiencies in terms of data traceability and audit preparation.
Industries
-
Pharmaceutical
Under continual pressure to increase profitability, maintain government compliance, and meet emerging market opportunities, the pharmaceutical manufacturing industry faces unique challenges that require unique solutions. As one of the world’s leading industrial automation suppliers, Yokogawa is poised and prepared to deliver those solutions, creating individualized lean manufacturing techniques that deliver consistent, measurable results.
Related Products & Solutions
-
OpreX Laboratory Information Management System
OpreX Laboratory Information Management System(hereinafter "OpreX LIMS") is a Laboratory Information Management System (LIMS) package, which supports quality management operations. Furthermore, it standardizes quality management operations to reduce costs and improve the service level.
Have Questions?
Contact a Yokogawa Expert to learn how we can help you solve your challenges.