Introduction
One of the primary applications for high purity water is boiler feed water. The measurement of pH can be one of the quickest indicators of process contamination in the production or distribution of pure water. Effective chemical treatment of the feed water is vital in maintaining a useful operating life and minimizing maintenance costs of the boiler. Boilers require pure water to reduce scaling and carryover of impurities in steam. Corrosion can occur when pH exceeds recommended limits at ranges that are dependent on metallurgies with the steam cycle.
One location for pH measurement, necessary to ensure that the chemical treatment is working effectively, is after the demineralizer. Since at this point, the water has almost no electrolytic conductivity, the measurement of pH is difficult. In steam cycle applications, pH can be measured at several locations including after water treatment, condensate pump discharge, after polishers if used, and boiler water. The measurement of pure water can lead to confidence that the water being used remains as pure as possible for the application.
Challenges
The low conductivity and limited buffering capacity of low ionic strength pure water causes the pH electrodes to drift. Results measurements are non-reproducible and inaccurate. In addition to large drift, common problems are unacceptable flow sensitivity and poor temperature compensation. Electrical noise and interference complicate matters further. Certain properties of pure water adversely affect the ability to obtain a reliable pH measurement. For many years, it was believed these properties could not be satisfactorily overcome in the pursuit of the desired measurement accuracy and reliability.
The areas most affected by there pure water properties include:
- Reference electrode stability
- Glass electrode response
- Electrical noise
- Special T.C. requirements
Reference Electrode
The liquid junction of the reference electrode tends to develop an appreciable diffusion potential as a result of the extremely large differences in concentration of ions between the process and the fill solution of the reference electrode.
The resulting junction potential can be as high as 20-40 millivolts (approximately 0.5 pH). Any change in this potential will show up as an erratic, drifting pH value.
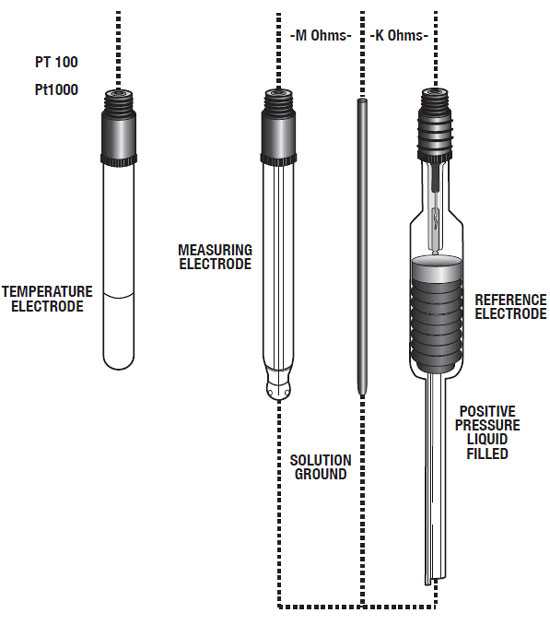
Figure 1: Typical Electrode Configuration for High Purity Water Applications
It will appear that there is a change in the process pH, but this change is false since it is caused by the junction potential (Figure 1). Depletion or dilution of the reference fill solution occurs much more rapidly in high purity water. That causes the reference potential to become unstable and the measurement unreliable.
Since there are no conductive ions to speak of in high purity water, a physical path of conductive reference solution from the reference electrode to the glass electrode must be established for the measurement circuit to be complete. If there are no ions provided from the reference electrode (ie. they have been depleted), there will be no stable reference from which to make the measurement.
Glass Electrode
The low ion concentration of pure water appears to hinder the glass pH bulb’s ability to detect hydrogen ions. This causes a low response speed in the electrode.
It is also possible that the alkali components of the glass measurement bulb may dissolve in pure water. If a low flow rate exists in the process, the result would be a pH reading that is too high.
Pure Water Flowing In a Pipe |
 |
| Other electrical sources such as group loop faults and electro- treatment processes will cause the same troubles. |
| Figure 2: Streaming Potentials |
Electrical Noise
Since pure water is a poor electrical conductor, it creates a static charge when flowing past non-conducting materials in the sensor. Pure water has a conductivity value of 0.055 µS (18.2 Mohm) at 25ºC. This liquid resistance can lead to the formation of surface static charges. This can generate “streaming potentials” (stray currents that can mimic pH) in the solution, which may cause large errors, or at least, excessive noise in the readings. A low impedance, well shielded and grounded electrode can reduce these errors to a minimal value, usually less than ±0.05 pH units. Since the electrical resistance of a typical measurement cell is so high, the electronics used to measure the cell potential are very susceptible to additional interfering factors such as extraneous electrical noise pickup and hand capacitance effects. These static charges, called streaming or friction potentials, are comparable to rubbing a glass rod (glass electrode) with a wool cloth (the water). This high resistance also increases the measurement loop’s sensitivity to surrounding electrical noise sources (Figure 2).
Another problem involves the buffering capacity of pure water, which is very low. When pure water is exposed to air the absorption of carbon dioxide (CO2) causes a decrease in the pH reading. Depending on temperature and pressure, the pH of pure water may drop to as low as 6.2. Taking grab samples to a lab meter should be avoided because atmospheric CO2 will contaminate the sample. Also, pure water temperature compensation must be taken into account.
Temperature Compensation |
 |
| Figure 3: Two major Forms of Temperature Compensation |
Temperature Compensation
There are two major temperature effects that must be addressed in order to establish a truly accurate representation of pH in high purity water. The standard automatic temperature compensator only corrects for one of these, often referred to as “Nernstian electrode correction.”
The second effect is known as the “equilibrium or dissociation constant correction.” While this effect is usually much smaller in magnitude, it can become significant. All solutions respond to changes in temperature in a specific manner (dissociation constant).
Depending on the solution, this response may be related to changes in pH or conductivity. The dissociation constant of pure water is 0.172 pH/10ºC. This means that at 50 ºC pure water has a pH of 6.61, while at 0 ºC it will have a pH of 7.47. The amount of temperature change involved and the critical nature of the measurement dictates whether this effect requires compensation (Figure 3).
Many of the problems associated with high purity pH can be reduced or eliminated through careful consideration of these critical aspects of the pH measurement loop.
Solutions
Through years of experience and innovative design, Yokogawa has developed solutions for the problems described earlier. The high diffusion potentials of the reference electrode can be overcome by using a positive pressure style electrode. One such electrode is the “Bellomatic” (Figure 1).
 |
| Figure 4: FF20 Configuration For High Purity |
Utilizing a large refillable reservoir, the electrode provides a constant flow rate of reference electrolyte. This provides for a longer, more economical service life than fixed reference electrodes could provide. In addition, the electrode is independent of the effects of process pressure. Therefore, the use of independent air pressure (as used with a salt bridge) is not required.
To counter the low response speed and the effect of the alkali components of the glass electrode, special low-impedance S-glass electrodes were developed. They have a chemically resistant glass texture and very good response time due to their low impedance.
An alternative to a separate glass and reference electrode is a combination electrode with the capability to pressurize the reference portion. In addition to the benefits already stated, the proximity of the two measuring elements helps insure electrode circuit continuity.
Noise problems resulting from ground loop potentials are addressed by the design of the pH transmitter. Many pH transmitters utilize a single-ended amplifier design. This design allows current (leakage current) to pass through the reference electrode, giving an offset in addition to shortening the useful life of the reference electrode. With the differential amplifier design, this leakage current will flow through the solution ground, not the reference. Therefore, no offset occurs and the reference electrode is not adversely affected.
Its magnitude is determined directly, using the Nernst Equation, which describes that the glass electrode operation is independent of the nature of the process fluid. The Nernst Equation simply states that as a glass electrode increases in temperature, its output voltage increases, even though the actual pH of the measured solution may remain the same. The effect is minimal at or near a pH of 7 and increases linearly above and below a pH of 7.
To prevent the increase of static potentials, a stainless steel flow chamber is recommended. Since most plastics are not completely gas tight, such a chamber will also prevent the absorption of CO2 from the air.
For accurate pH measurement:
- The sample temperature should preferably be in the 20° to 30°C range and remain constant.
- The sample must not be stagnant; otherwise, errors will result
- Constant flow rates between 50 ml and 150 ml provide the best results
- Air must not be allowed into the sample stream
- Temperature compensation for both the Nernst potentials and the dissociation constant of pure water is required.
It is also beneficial to measure pH in the smallest sample volume possible. Direct pH measurement in large volume samples such as drums or tanks and other samples with flowing or moving water tend to fluctuate and will require excessive stabilization time.
Summary
Measurement of pH in high purity water presents many difficulties. To achieve a successful measurement, care must be taken to address the unique problems of the application.
Selecting the proper electrodes and holder will eliminate problems with reference junction potentials, slow glass electrode response and surface static charges. Selecting the proper transmitter or analyzer will eliminate ground loop problems and allow for accurate temperature compensation for both the Nernst potentials and the dissociation constant of pure water. Sensor diagnostics enable the operator to ensure that the measurement loop is functioning properly.
Yokogawa offers the electrodes (Bellomatic reference and special G-glass measure electrode, or combination style); the sensor holder (model FF20/FS20 stainless steel flow-through style); and the transmitter or analyzer (Models PH450G/FLXA21 with sensor diagnostics and “process temperature compensation”) to provide accurate pH measurement in high purity water.
Where Are the Opportunities
While the major players in pure water pH applications are power plants, any facility that uses a boiler will need to monitor the pH in feed water. Pharmaceutical applications also demand pure water where it is used as an ingredient.
Product Recommendations
 |
| Figure 5: PH8HH Configuration for High Purity |
Measurement System
Process Liquid Analyzer:
2-wire FLXA202 pH/ORP Analyzer
4-wire FLXA402 pH/ORP Analyzer
Sensor Selection:
Option #1:
Holders
- FF20 Flow-thru assembly with individual measure, reference, and temperature electrodes
- FS20 Insertion assembly with individual measure, reference, and temperature electrodes
Electrodes
- Bellowmatic reference electrode (SR20-AC32), coupled with the shock-proof measuring electrode (SM21-AG4) and Pt1000 temperature electrode (SM60-T1)
Option #2:
Holder
- PH8HH Flow-thru assembly
Sensor
- PH8EHP Flowing reference pH Sensor for high purity water
Related Industries
-
Power
Since the 1970s, Yokogawa has partnered with power producers to squeeze every watt of efficiency and resilience from assets old and new. Today our 30,000+ CENTUM control systems and 230 service hubs span 100 countries, uniting domain know‑how, ISASecure™‑certified automation, and real‑time analytics. Whether you run a single gas turbine or a continent‑wide renewable fleet, we deliver the insight, safety, and speed you need to thrive in a decarbonizing grid.
-
Food & Beverage
The food and beverage industry must produce safe, high-quality foods and beverages for consumers. In addition to quality control, the manufacturing processes include many challenges such as managing ingredients, improving efficiency and handling global environmental issues. Yokogawa leverages its decades of technological expertise to help customers build and operate the ideal factory.
-
Pharmaceutical
Under continual pressure to increase profitability, maintain government compliance, and meet emerging market opportunities, the pharmaceutical manufacturing industry faces unique challenges that require unique solutions. As one of the world’s leading industrial automation suppliers, Yokogawa is poised and prepared to deliver those solutions, creating individualized lean manufacturing techniques that deliver consistent, measurable results.
Related Products & Solutions
-
2-Wire Transmitter/Analyzer FLXA202
Most flexible two-wire analyzer available
-
2-Wire Transmitter/Analyzer FLXA21
The FLEXA™ series analyzers are used for continuous on-line measurements in industrial installations. With an option for single or dual sensor measurement, they are the most flexible two-wire analyzer available.
-
Digital SMART SENCOM™ Adapter, SA11
Reusable SMART adapter, requiring only the analog sensor to be disposed of when it reaches the end of its lifetime. With the SENCOM 4.0 platform, Yokogawa delivers reduced costs and waste while contributing to its long-term business goals of a sustainable future for all.
-
Flow/NPT Fittings FF20/FS20
FF20/FS20 Fittings for pH and ORP
-
Industrial pH/ORP Electrodes
The heart of a pH measuring loop is the electrode system. Yokogawa has designed a wide range of electrodes to ensure this heart keeps beating under the most severe conditions.
-
Multi Channel 4-Wire Analyzer FLXA402
Available in single or multi-sensor measurement
-
pH and ORP Analyzers
Optimize field maintenance, calibration, and system configuration
-
pH and ORP Sensors
Ensure fluid process operations
Have Questions?
Contact a Yokogawa Expert to learn how we can help you solve your challenges.