Upcoming Events
-
Conference Sep 18 - 20, 2024 Boston, MASBI2
Join the Life Science team at SBI2! Meet with our industry experts at booth #12 as we showcase our CellVoyager CQ1 High-Content Analysis System. With clear 3D imaging, object recognition, and rapid quantification of live cells and cell clusters, the CQ1 offers a new approach to cell measurement. Ask one of our industry experts for a demonstration!
-
Tradeshow Oct 5 - 9, 2024 Chicago, ILNeuroscience 2024
At Yokogawa, we are committed to increasing throughput for faster, better research and results. Meet the Yokogawa team at booth #1559 at the 2024 Society of Neuroscience show (#SfN24).

A portfolio of flexible life science products and solutions for the regenerative medicine, pharmaceutical research, and precision medicine industries.
Yokogawa’s high content analysis systems and dual spinning disk technologies are known for higher product quality, more individualized systems tuning, and better process control. From design to implementation and startup to continuous optimization, Yokogawa has the experience and technology to solve your greatest challenges.
-
Spinning Disk Confocal CSU
- Yokogawa’s Spinning Disk Confocal Scanner Units (CSUs) real-time live-cell imaging
- Proprietary Microlens-Enhanced Dual Spinning Disk design
- Confocal Super Resolution with SoRa unit
-
High-Content Analysis Systems
Utilizing powerful software, high-content analysis (HCA) systems address a wide range of research applications from basic science to complex compound screening.
-
FlowCam: Flow Imaging Microscopy
By analyzing particles accurately, reliably, and quickly via automated imaging, FlowCam® technologies advance research, increase productivity, and ensure quality.
-
Single-Cell Analysis Solutions Single Cellome™
We are developing cell handling technology for single-cell and live cells. SU10 provides selective, minimally damaging automated nano-point delivery. SS2000 provides automatically subcellular sampling based on confocal microscopy technology.
Details
Principles of Spinning Disk Confocal
The most common conventional confocal microscopes use a single laser beam to scan a specimen, while the CSU scans the field of view with approximately 1,000 laser beams by using microlens-enhanced Nipkow-disk scanning. In short, CSU can scan 1,000 times faster.
By using a disk containing microlens arrays in combination with the Nipkow disk, we have succeeded in dramatically improving the light efficiency and therefore successfully made real-time confocal imaging of live cells possible.
The expanded and collimated laser beam illuminates the upper disk containing about 20,000 microlenses (microlens array disk). Each microlens focuses the laser beam onto its corresponding pinhole, thus effectively increasing laser intensity through pinholes placed in the pinhole array disk (Nipkow disk).
With the microlens, backscattering of laser light at the surface of the pinhole disk can be significantly reduced, dramatically increasing the signal to noise ratio (S/N) of confocal images.
About 1,000 laser beams passing through each of the pinholes fill the aperture of the objective lens, and are then focused on the focal plane. Fluorescence generated from the specimen is captured by the objective lens and focused back onto the pinhole disk, transmitted through the same holes to eliminate out-of-focus signals, deflected by the dichroic mirror located between microlens array disk and the Nipkow disk to split fluorescence signal from reflected laser, passed through emission filter and then focused into the image plane in the eyepiece or camera.
The microlens array disk and the Nipkow disk are physically fixed to each other and are rotated to scan the entire field of view at high speeds, making it possible to view confocal fluorescent images in real-time through the eyepiece of the CSU head.
Compared to conventional single point scanning, multi beam scanning by the CSU requires a significantly low level of light intensity per unit area, which results in significantly reduced photo bleaching and phototoxicity in live cells.
Spinning Disk Confocal

Microlens-enhanced Nipkow Disk Technology

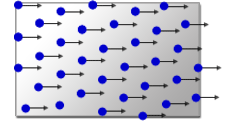
Comparison of scanning method

Point Scanning
1 line scan time=1[ms]
1000 lines/image
Scan lines=1000 [lines]
1×1000=1000 [ms]
Disk Scanning by CSU
Rotation Speed=10000 [rpm]=41.7[rps]
30°Rotation/image
1÷( 41.7×30/360 )= 0.5 [ms]
What is a Confocal Scanner Unit?
Confocal scanner unit CSU series enable 3D observation of the cells in detail and dynamics of organelles inside cells. Since the CSU series is capable of high-speed shooting, it is also suitable for observing high-speed life phenomena. In addition, the CSU series is a multi-point confocal method which is extremely gentle to cells, best suitable for long-term live cell observation.
Industrial Applications & Types of Confocal Scanner Units
For pharmaceutical, food, and cosmetic developers
- Evaluation of drug efficacy and toxicity by using cultured cells instead of expensive animal experiments
- Evaluation experiments using cell clusters (spheroids, organoids), which have been extensively researched in recent years
- Evaluation of effects of functional foods on cells
- Efficacy evaluation of cosmetics by using 3D skin model
- Confirmation of stem cell differentiation state and quality evaluation for regenerative medicine

Benefits of Confocal Scanner Units
Yokogawa spinning disk confocal technologies:
- High-efficiency imaging
- Less photobleaching and phototoxicity
- Less expensive
CSU- W1:
- FOV is 4x wider than other conventional industry models
- Capable of fully automated experiments
- Multiple configurations
- Selectable pinhole size
CSU-X1:
- World's fastest scanning speed of up to 2,000 fps
- Microlens-enhanced Nipkow disk scanning
- Exchangeable dichroic mirror block and emission filters
- O2 emissions reduced by 40%
Resources
Visualizing the cell behavioral basis of epithelial morphogenesis and epithelial cancer progression
Faster, Deeper, and Clearer -in vivo molecular imaging technology-
Discovering the Basic Principles of Life through the Live Imaging of C. elegans
Closing in on Neuronal Circuit Dynamics through High-speed, fMCI.
New Era in Manmmalian Genetics Research: To utilize the same embryo after long-time 3D observation!
Getting Closer to “Plant Cell World”with High-speed Live Imaging and Image Information Processing.
First annual Yokogawa CSU Spinning Disk Image Competition at MBL 2023
Use of the spinning disk confocal at the Harvard Medical School microscopy core.
Spinning Disk Confocal Microscopy for Quantitative Imaging and Multi-Point Fluorescence Fluctuation Spectroscopy.
drug discovery and drug development for an interrelated set of disorders that emanate from type II diabetes and obesity
On-site manipulation of protein activities: Understanding intricate cell signaling pathways.
world-class team with an unmatched combination of imaging experts, assay development experience and the latest technologies and data analysis capabilities
single-cell analysis specifically transcription by steroid receptors primarily estrogen and androgen receptors
In recent years, single cell analysis has become increasingly popular due to the new development of advanced and high sensitivity analytical methods.
SS2000 is a revolutionary system that can sample subcellular components and a whole cell by using a glass capillary tip with an inner diameter of a few μm while imaging with a confocal microscope.
This application note provides an example of single cell RNA sequencing scRNA seq) from cells sampled by SS2000, and the data is comparable to conventional methods.
In recent years, research on single cells has been actively conducted.However, with more sensitive analytical techniques, it is now possible to analyze specific intracellular components at the single-cell level.
The SS2000 is a revolutionary system that allows both confocal imaging and sampling of targeted intracellular components or single cells using a glass tip with an inner diameter of a few micrometers. The SS2000 includes an incubator as well as highcontent imaging functions, such as long time-lapse observation, machine learning, and label-free analysis.
The SU10 is an innovative device that delivers target substances directly into cells or into nuclei with a nanopipette that has a tip outer diameter as small as a few tens nm.
This application note shows a case study of sampling and analysis with the SS2000 from cells delivered intracellularly with the SU10.
In recent years, research on single cells has become increasingly popular. With more sensitive analytical techniques, it has become possible to analyze specific intracellular components such as organelles, even at the single-cell level.
The SS2000 is an innovative system that can sample subcellular intracellular components using a glass capillary having a tip diameter of only a few micrometers while imaging with a confocal microscope.
In this application note, we introduce a case where after drug treatment, intracellular components were sampled by the SS2000 and analyzed by single-cell mass spectrometry.
In recent years, single-cell studies have become increasingly popular, leading to the development of instruments capable of spatial omics - the integration of samples' 3D locations and their omics data. The SS2000 is a revolutionary device that can collect intracellular components at the single-cell level and image the samples using a confocal microscope. In this application note we introduce the research performed by Dr. Okada of The University of Tokyo, who established an intra single living cell sequencing (iSC-seq) method which uses SS2000 to sample intracellular components from osteoclasts (a type of multinucleated giant cell) and analyzes them through next-generation sequencing.
Comparison between CSU and conventional LSM in 4D movies.
The CV8000 nuclear translocation analysis software enables the analysis of changes in the localization of signal molecules that transfer between cytoplasm and nuclei, such as proteins. The following is an example of the translocation analysis of NFκB, a transcription factor.
A critical requirement in biopharmaceutical development is the integration and automation of process equipment and analytical instruments used in the laboratory. Bioprocess labs with multiple lab-scale bioreactors often execute cultivation experiments in parallel for research or process development purposes.
As part of a collaboration between Securecell (Zurich, SW) and Yokogawa Life Science (Tokyo, Japan), this application note demonstrates the effective use of the Lucullus® Process Information Management System (Lucullus®) to assist in the control of three Advanced Control Bioreactor Systems (BR1000) to study glucose utilization of CHO cells for optimal monoclonal antibody productivity.
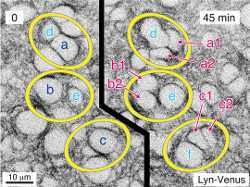
To investigate interactive dynamics of the intracellular structures and organelles in the stomatal movement through live imaging technique, a CSU system was used to capture 3-dimensional images (XYZN) and time-laps images (XYT) of guard cells.
Cell stage categorized using FucciTime lapse imaging of Fucci-added Hela cells was conducted over 48 hrs at 1 hr intervals. Gating was performed based on the mean intensities of 488 nm and 561 nm for each cell. They were categorized into four stages, and the cell count for each was calculated.
The SU10 is a novel technology that can deliver target substances into cells (nucleus or cytoplasm) by using a "nano" pipette made of glass capillary with an outer tip diameter of just tens of nanometers.
The CQ1 confocal image acquisition mechanism with the distinctive CSU® unit has a function to sequentially acquire fine cell images along the Z-axis and capture information from the entire thickness of
cells which include heterogenic populations of various cell cycle stages. In addition, saved digital images can be useful for precise observation and analysis of spatial distribution of intracellular molecules.
The CQ1 capability to seamlessly analyze images and obtain data for things such as cell population statistics to individual cell morphology will provide benefits for both basic research and drug discovery
targetingM-cell cycle phase.
Applications: Colony Formation, Scratch Wound, Cytotoxicity, Neurite Outgrowth, Co-culture Analysis, Cell Tracking
Faster, Brighter, and More Versatile Confocal Scanner Unit
Welcome to The New World of High Content Analysis
High-throughput Cytological Discovery System
In recent years, research on single cells has become increasingly popular, but with more sensitive analytical techniques, it has become possible to analyze specific intracellular components such as organelles at the single-cell level.
The SS2000 is an innovative system that can sample intracellular components at the single-cell level using a tip (glass capillary) with a diameter of several micrometers while imaging with a confocal microscope. In this application note, we introduce a case in which intracellular components were sampled by SS2000 and genetic analysis was performed by qPCR.
This application note will introduce the features of the SU10 and provide examples demonstrating the delivery of genome editing tools (Cas9 RNP) using the technology.
SU10 is a novel technology that enables the delivery of target substances directly into cells (nucleus or cytoplasm) using a "nano" pipette made of a glass capillary with an outer tip diameter of tens of nanometers.
Fluorescent ubiquitination-based cell cycle indicator (Fucci) is a set of fluorescent probes which enables the visualization of cell cycle progression in living cells.
Yokogawa collaborates with scientists in the medical and pharmacology fields to identify best practices for cell painting and high-content screening, thereby enhancing image analysis and reproducibility.
List of Selected Publications for the Wide Field of View Confocal Scanner (CSU-W1)
This page shows the list of Selected Publications on the Benchtop Confocal System CQ1
List of Selected Publications for the High-Speed Confocal Scanner (CSU-X1)
List of Selected Publications for High-Content Screening Systems: CV8000, CV7000, CV6000
This "Tutorial" provides overview of this software, from installation through data analysis.
In this tutorial, a method for analyzing ramified structure, using CellPathfinder, for the analysis of the vascular endothelial cell angiogenesis function will be explained.
In this tutorial, a method for analyzing ramified structure, using CellPathfinder, for the analysis of the vascular endothelial cell angiogenesis function will be explained.
In this tutorial, spheroid diameter and cell (nuclei) count within the spheroid will be analyzed.
In this tutorial, we will learn how to perform time-lapse analysis of objects with little movement using CellPathfinder, through calcium imaging of iPS cell-derived cardiomyocytes.
In this tutorial, we will identify the cell cycles G1-phase, G2/M-phase, etc. using the intranuclear DNA content.
In this tutorial, image analysis of collapsing stress fibers will be performed, and concentration-dependence curves will be drawn for quantitative evaluation.
In this tutorial, we will observe the change in number and length of neurites due to nerve growth factor (NGF) stimulation in PC12 cells.
In this tutorial, intranuclear and intracytoplasmic NFκB will be measured and their ratios calculated, and a dose-response curve will be created.
In this tutorial, we will learn how to perform cell tracking with CellPathfinder through the analysis of test images.
In this tutorial, using images of zebrafish whose blood vessels are labeled with EGFP, tiling of the images and recognition of blood vessels within an arbitrary region will be explained.
Downloads
Brochures
- CellVoyager CQ1 Benchtop High-Content Analysis System (2.9 MB)
- Life Innovation (2.4 MB)
- Yokogawa in the Pharmaceutical Industry (7.7 MB)
- CellVoyager CV8000 High-Content Screening System (2.5 MB)
- Yokogawa CSU-W1 SoRa Confocal Scanner Unit (525 KB)
- CSU-X1 Confocal Scanner Unit (2.4 MB)
- FlowCam 8000 Imaging Particle Analysis System (720 KB)
Videos
Fast, gentle, and clear - live-cell imaging. Yokogawa's unique scanning method minimizes damage to living cells and organisms and even can capture faint/fast life phenomena.
More than 2,500+ units scanning units sold worldwide. This fast, reliable, and accurate technology has been leading cutting-edge research and supporting researchers around the world for more than two decades.
More information: https://www.yokogawa.com/us/solutions/products-platforms/life-science/spinning-disk-confocal/
#confocal #microlens #microscope #CSU #Yokogawa #livecell
Yokogawa's CQ1 open platform integrates seamlessly with Advanced Solutions BioAssemblyBot® 400. With laboratory automation becoming a standard in research, Yokogawa's high content confocal system's ability to work with robots like Advanced Solutions' BioAssemblyBot® 400 is essential to advancing laboratory automation.
Welcome to a transformative journey in the pharmaceutical industry!
Today, we're diving into how we, Yokogawa are reshaping the pharmaceutical landscape. Our focus? Innovation and digitalization. This isn't just about technology; it's about ensuring a stable, reliable supply of medicines to those who need them most.
We're tackling key industry challenges: enhancing flexibility and agility, reducing time to market, and boosting process efficiency.
How? By harnessing the power of data. Join us as we explore how Yokogawa's cutting-edge solutions are making "right-first-time" and "zero-waste" drug manufacturing a reality. From on-premise to cloud-based solutions, we're bringing smarter, more secure, and more cost-effective production to life. Let's uncover the future of pharmaceuticals, powered by Yokogawa!
For more information: https://www.yokogawa.com/us/industries/pharmaceutical/
3D imaging experts from Yokogawa and Insphero have come together to provide helpful tips and tricks on acquiring the best 3D spheroid and organoid imaging. This webinar focuses on sample preparation, imaging, and analysis for both fixed and live cells in High Content Screening assays. The experts also discuss automated tools that can help researchers understand the large volume of data in these High Content Imaging Analysis Systems.
In this webinar, Professor Jonny Sexton discusses a pipeline, developed in the Sexton lab, for the quantitative high-throughput image-based screening of SARS-CoV-2 infection to identify potential antiviral mechanisms and allow selection of appropriate drug combinations to treat COVID-19. This webinar presents evidence that morphological profiling can robustly identify new potential therapeutics against SARS-CoV-2 infection as well as drugs that potentially worsen COVID-19 outcomes.
Physiologically relevant 3D cell models are being adopted for disease modeling, drug discovery and preclinical research due to their functional and architectural similarity to their tissue/sample of origin, especially for oncology research. Multifunctional profiling and assays using 3D cell models such as tumoroids tend to be manual and tedious. Further, high-content imaging of biomarkers in 3D cell models can be difficult.
In this two-part webinar present to you streamlined technologies which can bring consistent timesaving, ease-of-use, and high-quality data to your 3D cell-based workflows:
(A) The Pu·MA System is a microfluidics-based benchtop automated device for performing “hands-off” 3D cell-based assays. In this webinar, application scientist Dr. Katya Nikolov will present data from optimized assays using tumoroids followed by Yokogawa’s high-content imaging systems for biomarker detection.
(B) Yokogawa’s high-content imaging systems such as CellVoyager CQ1 provide superior confocal imaging using the Nipkow Spinning Disk Confocal Technology. Here, application scientist, Dan Collins will present details of the high-content imaging capabilities, easy to use and intuitive image acquisition software, especially for increasing productivity and a streamlined workflow.
Learn How:
- The open platform, Pu·MA System can be used to automate your 3D cell-based assays
- To perform automated IF staining for biomarkers using tumoroid models without perturbing your precious samples
- Image acquisition from 3D cell models using Yokogawa’s high-content imaging platforms
- Image analysis from cells, complex spheroids, colonies, or tissues using the CellPathfinder high content analysis software
Physiologically relevant 3D cell models are essential for drug discovery and preclinical research due to their functional and architectural similarity to solid tumors. One of the challenges faced by researchers is that many of the assays using these precious samples tend to be manual and tedious.
Using proprietary microfluidics technology, Protein Fluidics has created the Pu·MA System for automated complex 3D cell-based assays. In this webinar, application scientist Dr. Katya Nikolov will present her work on combining this novel automation technology with Yokogawa’s high-content imaging systems for biomarker detection in 3D cell models. Nikolov will demonstrate the utility of an automated immunofluorescence staining workflow followed by confocal imaging within the Pu·MA System flowchips. This automated workflow enables quantitative assessment of biomarkers which provides valuable data for further understanding disease mechanisms, preclinical drug efficacy studies, and in personalized medicine.
This webinar will explore:
- The Pu·MA System and novel technology for automated 3D cell-based assays
- How to perform automated immunofluorescence staining for biomarkers with a “hands-off” assay workflow
- How to visualize biomarkers after the assay with high-content imaging within the flowchip
Visualizing the complex spatiotemporal dynamics of human stem cells as they proliferate and make cell fate decisions is key to improving our understanding of how to robustly engineer differentiated tissues for therapeutic applications.
In this webinar, Dr. Rafael Carazo Salas will describe multicolor, multiday high-content microscopy pipelines that his group has recently developed to visualize the dynamical cell fate changes of human Pluripotent Stem Cells (hPSCs).
Key Topics:
- Visualizing how human Pluripotent Stem Cells (hPSCs) proliferate and undergo early differentiation in vitro, by high content microscopy
- Learning about experimental and computational pipelines that enable monitoring single-cell fate dynamics
- Learning about novel “live” reporters of hPSC cell fate
Speaker
Rafael Carazo Salas, PhD
Professor, School of Cellular and Molecular Medicine
University of Bristol
This webinar highlights Yokogawa’s High Content Solutions, the benchtop confocal CellVoyager CQ1, and CellVoyager CV8000. Utilizing Yokogawa’s dual-wide microlens spinning disk confocal technology, these automated HCA systems provide remarkable image quality while increasing your output. This frees up time to complete other research activities. Also, recent additions to the CSU-W1 confocal upgrade is discussed. The SoRa, a super-resolution solution, and the Uniformizer, an image flattening device. Both of which can be added to the lightpath of your CSU-W1-enhanced microscope.
Agenda:
Introduction to Yokogawa
SoRa for CSU-W1 super-resolution with confocal
Two high content instruments from Yokogawa: The CQ1 and the CV8000
Presenter:
Dan J. Collins, Applications Scientist, Yokogawa Life Science
Image-based phenotypic screening relies on the extraction of multivariate information from cells cultured in a large number of screened conditions. In this webinar, we explored the application of complex and biologically relevant model systems for drug screening, such as small intestinal organoids.
Key topics include:
- Learn how to upscale, streamline, and automate intestinal organoid handling
- Learn how to image in complex three-dimensional (3D) model systems and how to approach large imaging datasets
- Understand the basics of multivariate analysis on image-inferred features
In the last few decades, the pharmaceutical industry has transformed people’s lives. However, the development of new drugs is becoming increasingly difficult and a paradigm shift in the drug discovery workflow is required to reduce attrition and transform conventional drug screening assays into translatable analytical techniques for the analysis of drugs in complex environments, both in-vitro and ex-vivo. The ability to visualize unlabelled compounds inside the cell at physiological dosages can offer valuable insight into the compound behavior both on and off-target.
SiLC-MS is a semi-automated methodology that allows the collection of intracellular contents using a modified CQ1 imaging system developed by Yokowaga. The instrument is equipped with a confocal microscope that allows bright field imaging as well as fluorescence imaging with 4 lasers (405, 488, 561, and 640 nm). Sampling is performed using the tips developed by Professor Masujima (1-4).
In this study, we show the applicability of the SiLC-MS technology to drug discovery, as it is crucial to identify compound and its metabolites when incubated in a mammalian cell at a therapeutic dose. We report on the validation studies performed using the SiLC-MS platform, in these validation studies we assess the ability to distinguish different cell types based on their metabolomic fingerprint, furthermore, we have also evaluated if this assay was sensitive enough to detect drugs intracellularly.
Presenter: Carla Newman, Scientific Leader (Celluar Imaging and Dynamics), GSK
Generating translatable high-content imaging data from physiologically-relevant cell models, including 2D and 3D structures, is extremely valuable for drug discovery and pre-clinical research. In this webinar, James Evans, CEO of PhenoVista Biosciences presents case studies on how Yokogawa’s Benchtop CQ1 Confocal System can improve throughput and standardize processes for complex 3D cell-based phenotypic assays.
Key learning objectives:
- Strategies for designing and implementing high-content screening assays
- Approaches for deciding between 2D and 3D model systems
Are you looking to improve laboratory workflows, data management, and imaging analysis?
This webinar covers the integration of a high-content imager into a modern laboratory automation system and workflows built to utilize it. This on-demand webinar describes the integration topology used in the High Throughput Bioscience Center at St. Jude and the technical challenges that emerged pertaining to data handling and analysis. The webinar addresses the variety of ways we have used our high-content imager in the context of a high throughput screening center, using examples of experiment workflows from recent users.
Key Topics:
- Body Copy:
- Key considerations for integrating a high-content imager into a laboratory robot system
- Methods for interfacing between robots and the imager
- Important considerations for imaging data management and analysis
- How the St. Jude High Throughput Bioscience Center supports the diverse imaging needs of its projects
Human pluripotent stem cells are proven efficient models for drug screening campaigns. They provide access to unlimited starting material amenable to high throughput small molecule compound screening. Due to their capacity to generate mode complex cellular models, they also offer the potential to perform high content screening in tissue-specific organoids for further human target validation.
Dr. Alejandro Hidalgo-Gonzalez at MCRI(Murdoch Children's Research Institute) in Australia is a user of our HCA system CellVoyager CV8000 and he has established a pipeline for assay development and automated unbiased phenotypic drug screening using human pluripotent stem cell-derived cells and organoids. This is a recording of his presentation at an educational webinar organized by A*STAR in Singapore.
News
-
News Brief Apr 25, 2024 Yokogawa Helps to Revolutionize the Field of Single-Cell Lipidomics
-
Press Release Jan 31, 2024 Yokogawa Introduces CellVoyager High-Content Analysis System CQ3000
- For greater efficiency in drug discovery and regenerative medicine R&D, and the swift commercialization of new drugs -
-
Press Release Jun 21, 2023 Yokogawa to Release OpreX Informatics Manager, Enabling Integrated Management of Experimental Data and Research Resources in the Cloud
-
Press Release Nov 30, 2021 Yokogawa Develops Single Cellome System SS2000 for Subcellular Sampling
-
Press Release Dec 3, 2020 Yokogawa and InSphero Enter into Partnership Agreement
- Supporting drug development research through the use of HCA and three-dimensional culture models -
Looking for more information on our people, technology and solutions?
Contact Us