Reduce Cycle Time. Increase Results.
Under continual pressure to increase profitability, maintain government compliance, and meet emerging market opportunities, the pharmaceutical manufacturing industry faces unique challenges that require unique solutions. As one of the world’s leading industrial automation suppliers, Yokogawa is poised and prepared to deliver those solutions, creating individualized lean manufacturing techniques that deliver consistent, measurable results.
Details
Yokogawa's Pharma 4.0 Solutions
Developed by the ISPE as a roadmap to introduce Industry 4.0 (also known as Smart Factory), Pharma 4.0 creates digital strategies for pharmaceutical manufacturing’s unique needs. In other words, it future-proofs productivity by increasing connectivity, simplifying compliance, and consolidating production information, allowing users to respond to problems in real-time.
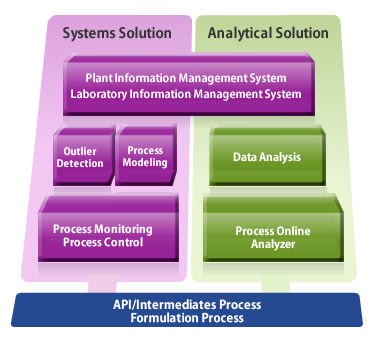
|
Data Analysis |
Monitoring |
Rapid Response |
Process Improvement |
Yokogawa Solution List

What Is Environmental Data Monitoring?
The Oprex Environmental Monitoring System collects, measures, and stores management data from pharmaceutical manufacturing, quality control, and storage areas, giving users the flexibility of multiple applications with the convenience of a single system.
• Data Integrity meets ALCOA+ requirements
• Long-term storage of collected data in recorders that are FDA 21 CFR Part 11 compliant
• Data monitoring in real-time
• Centralized management of usernames and passwords
Benefits Of Environmental Data Monitoring
Designed to monitor environmental data in real time, the Oprex Environmental Monitoring System alerts users to problems before they can affect product quality or turnaround. The collected data is stored in recorders that are FDA 21 CFR Part 11 compliant and the data integrity meets ALCOA+ requirements. In addition, the centralized user management system makes it convenient to control logins, passwords, and user access, creating audit trails and supporting up to 7 languages.
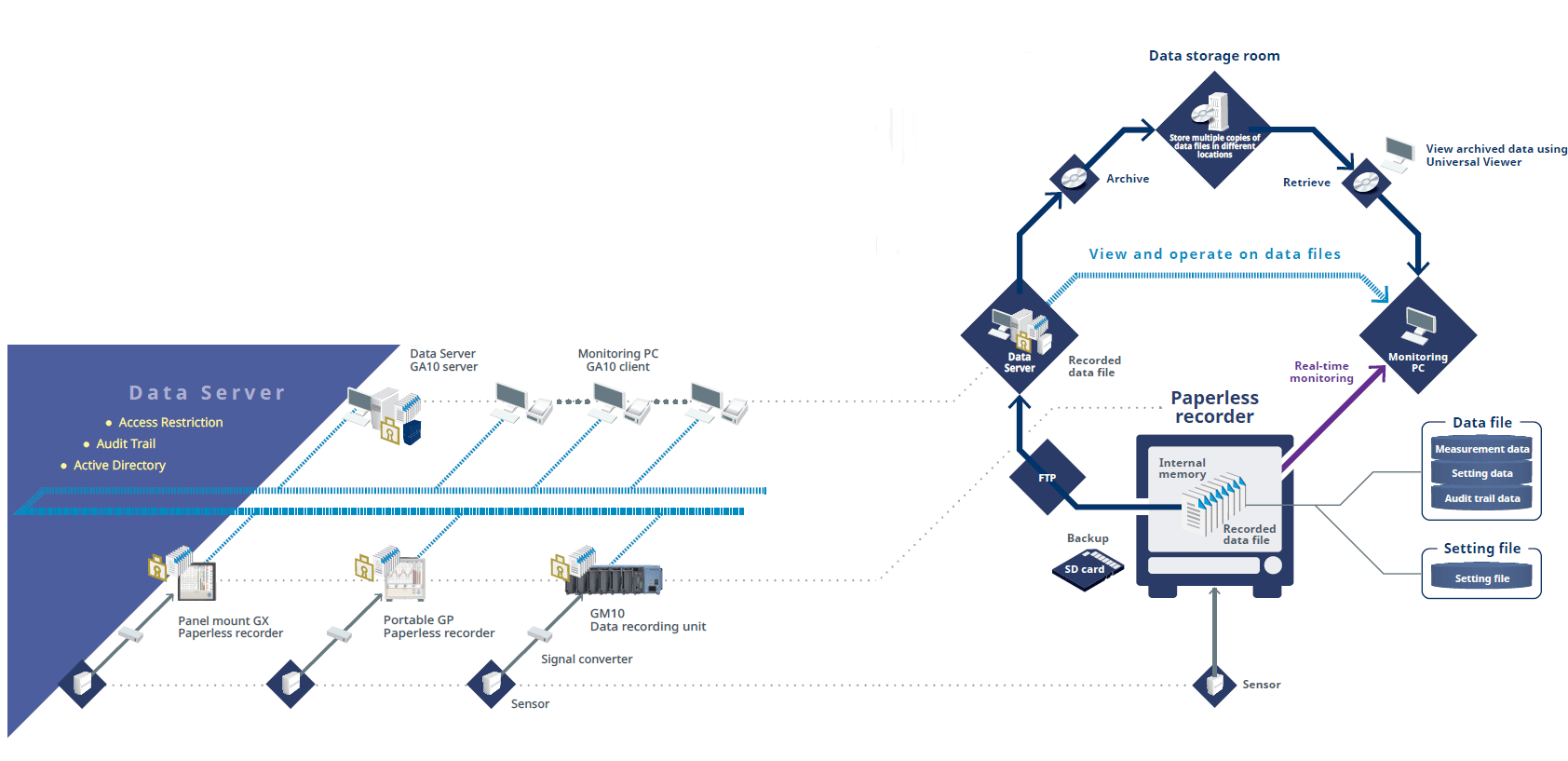
The Oprex Environmental Monitoring System Features
Access Control
Users are assigned to groups and are given access privileges based on the group they belong to.
Only authorized users can login to the system and search and browse through data.
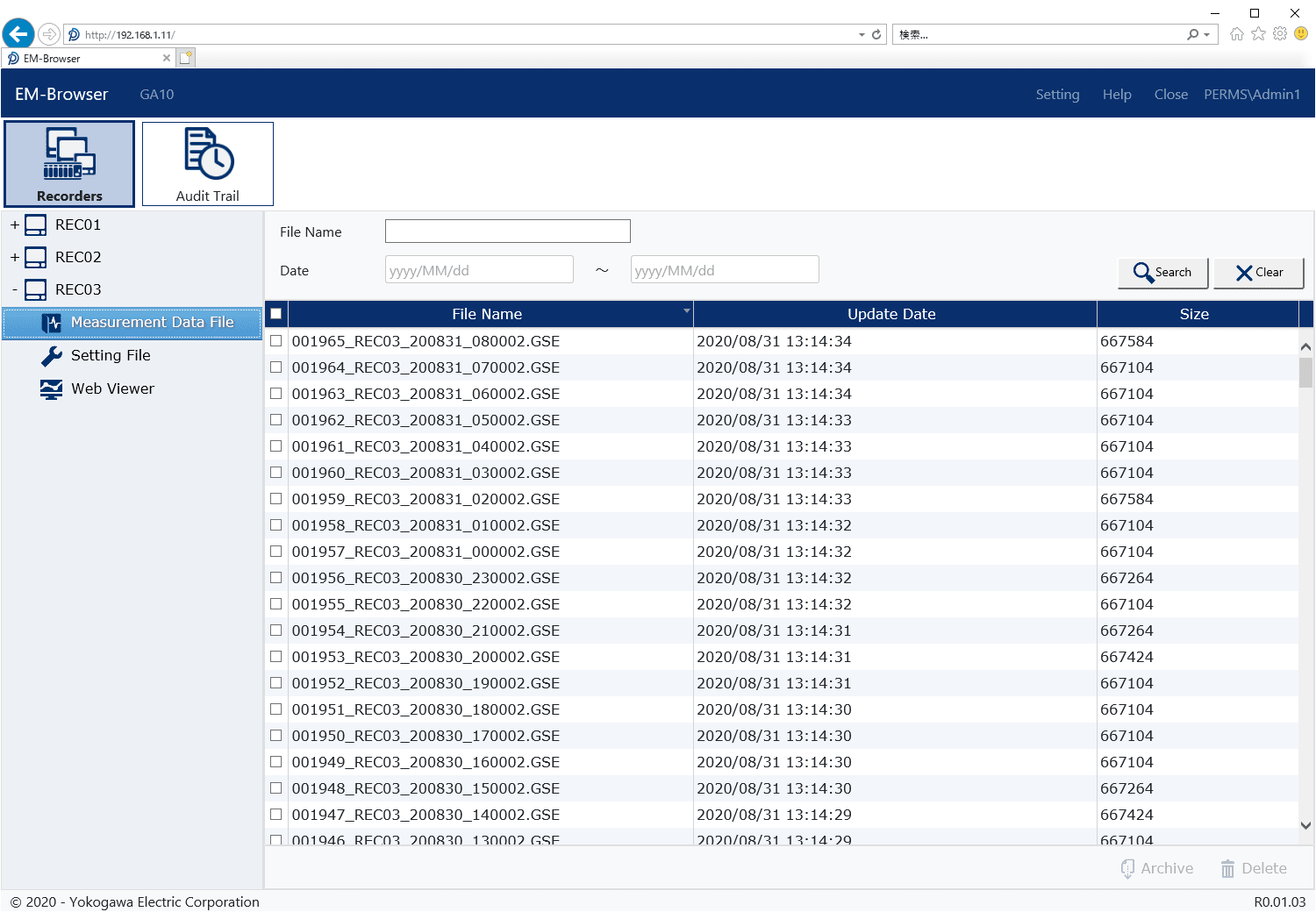
Audit Trails
All file operations by an authorized user are recorded in the audit trail.
- File operations (Archive, Delete)
- Backup and Restore
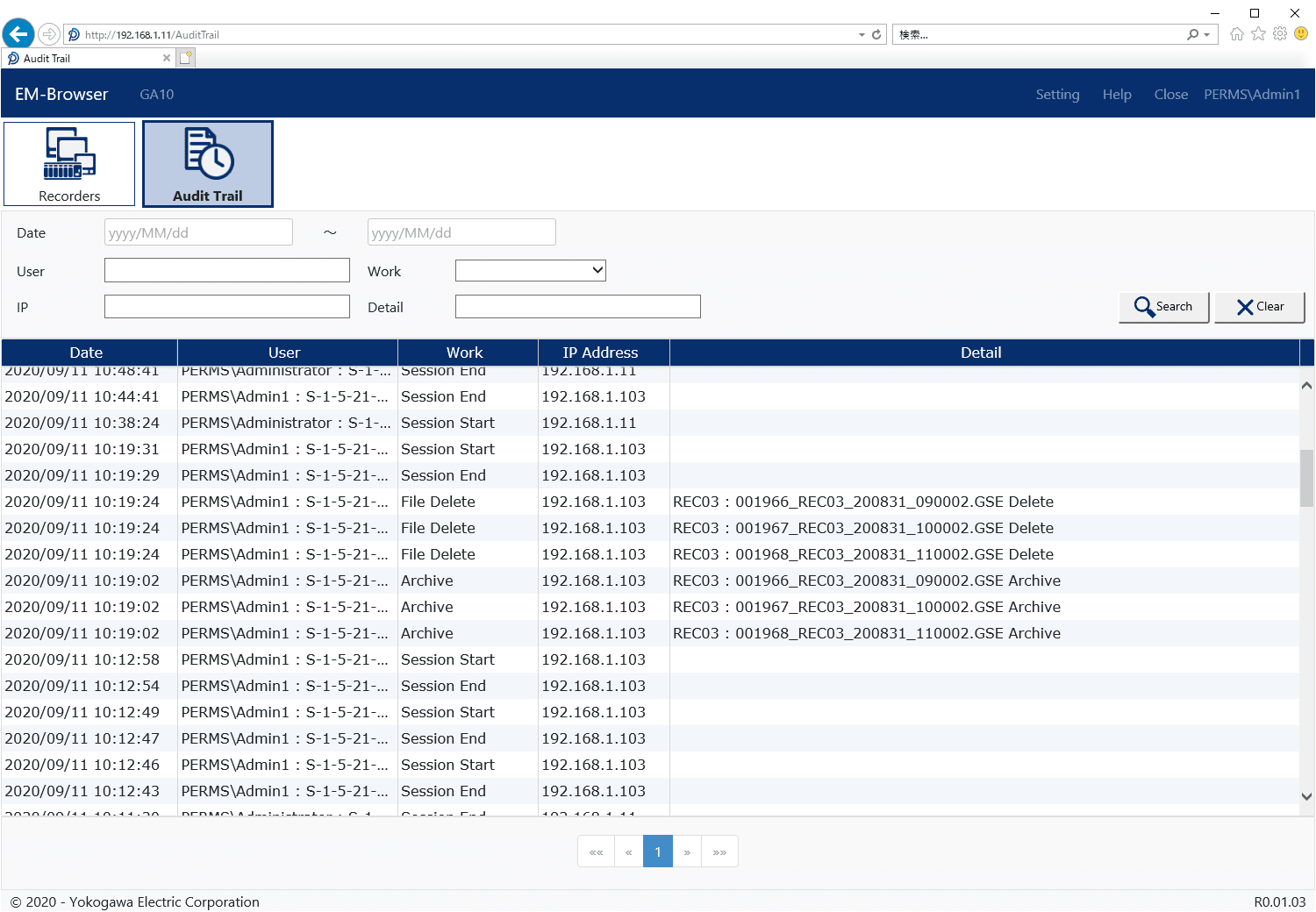
Configuration Tool
Adminstrator configures the Data Management Package using this tool.
- Register new recorders
- Register new users
- License management
Monitor measured data in real time
- Measured value display (Trend display / Digital display)
- Alarm notification on the screen
- Monitor data for each measurement group

Dedicated viewer software for browsing recorded data
- Display recorded data
- Supports latest OS and offers best viewing quality
- Supports 7 languages
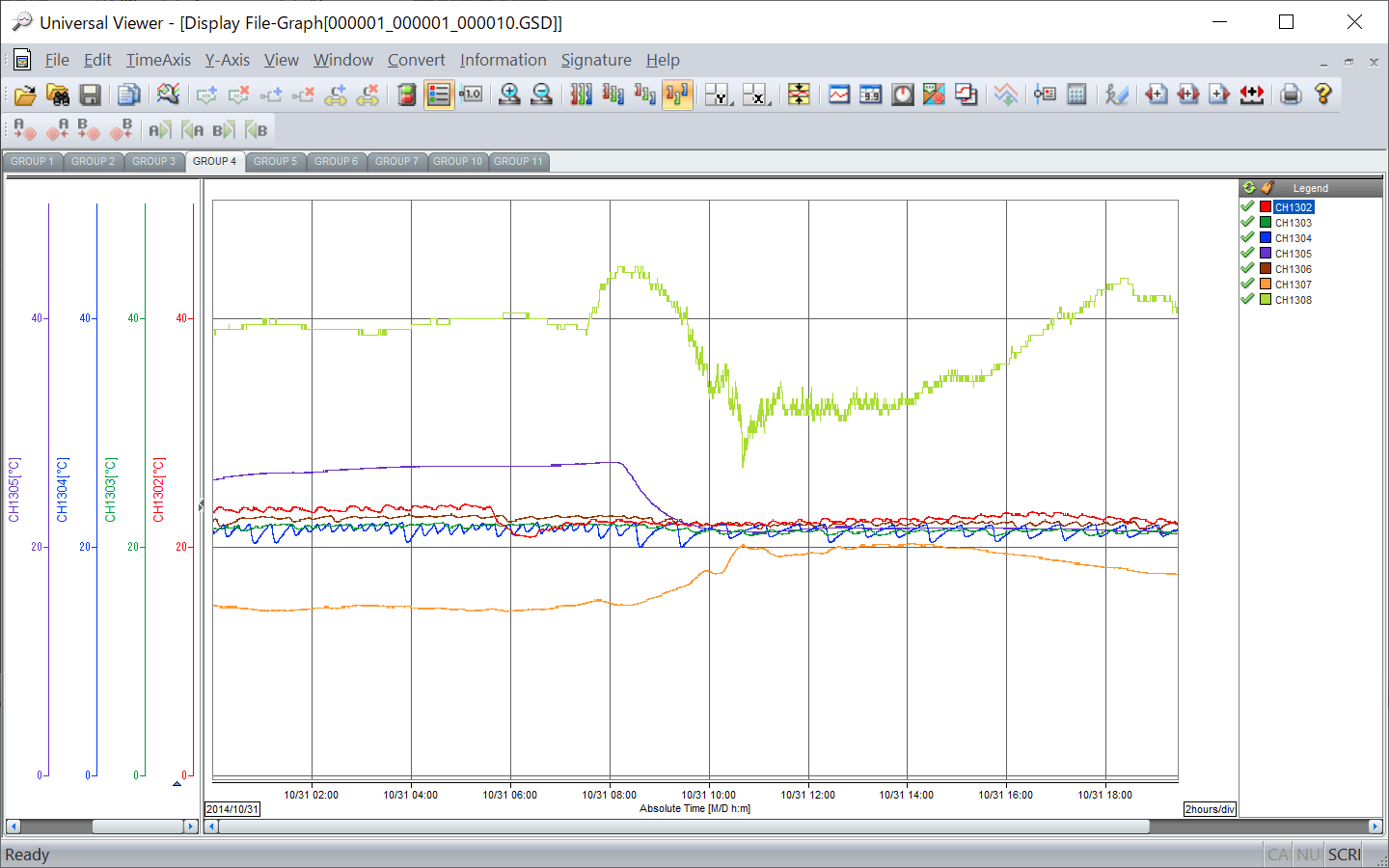
Paperless Recorder
SMARTDAC+ Series
Measure and record a variety of data
- Add I/O modules as needed
- Wide variety of powerful display functions
- A full range of network functions
Advanced Security Function
Electronic recording of data is compliant with pharmaceutical regulations
- Logical security
- Audit trail function
- Secure data storage in binary format (fraud proof)
- Dedicated viewer software
Why Lean Manufacturing?

Today’s pharmaceutical manufacturers face ever-increasing challenges. Caught between the demand for low cost drugs and the rising costs of production, they must improve efficiency and productivity without sacrificing quality or turnaround. At Yokogawa, we understand these challenges, and more importantly, we understand that Lean Manufacturing is the answer. Through the strategic use of our automation solutions, innovative process controls, and global network of experts, customers have optimized their production lifecycles, anticipating and correcting issues before they become problems.
Lean Manufacturing Solutions
Yokogawa’s portfolio of Lean Manufacturing solutions enable plantwide integration and product lifecycle optimization, increasing efficiency and productivity.

The CENTUM VP, Integrated Production Control System
FDA 21 CFR Part 11 compliant and capable of supplying batch functions based on the ISA-88 batch process control standard, the CENTUM VP, Integrated Production Control System tracks production trends in real-time, connecting a plant’s various interacting units and automatically addressing problems as they arise.

The SMARTDAC+® GX/GP Series Paperless Recorders
Meeting the requirements of FDA 21 CFR Part 11, the SMARTDAC+® GX/GP Series Paperless Recorders are capable of all essential validation documents, including stored data electronic signatures.

The FA-M3V, IT Machine Controller
A high-performance, programmable logic controller (PLC), the FA-M3V, IT Machine Controller combines rapid processing speeds with stable control features, ensuring consistent product quality.
Customer Challenge

Compliance with globalized regulations and guidelines
Pharmaceutical companies operate in a global marketplace and the industry is encouraged to comply with international initiatives such as the PIC/S GMP Guide and the ICH Guidelines. GMP facilities need to follow established best practices and automated production equipment and computer systems are expected to adopt current computerized system validation (CSV) practices such as GAMP.
Our Solutions
Assuring the Production of Quality Pharmaceutical Products
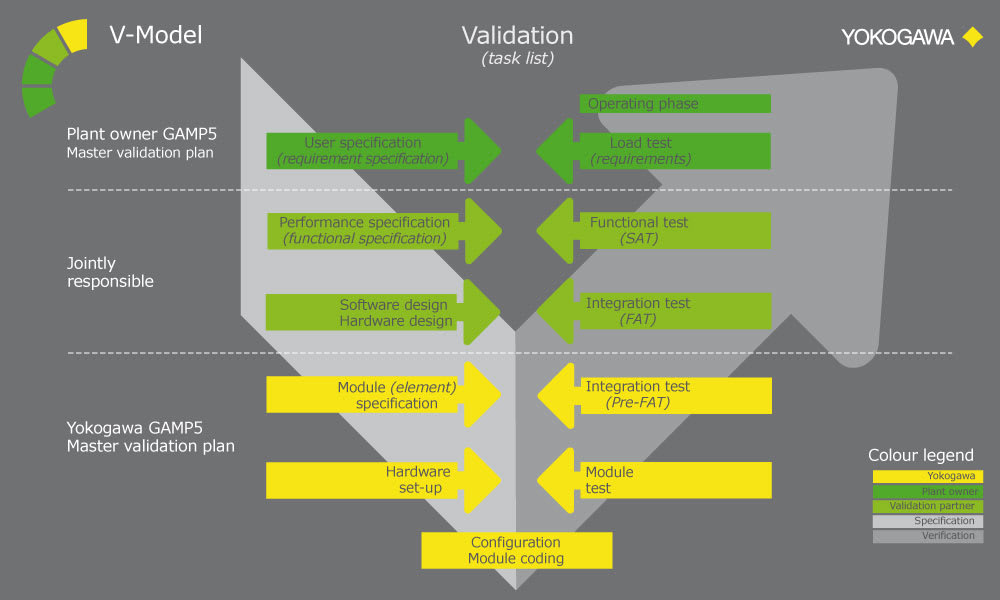
Over the years, Yokogawa has successfully realized numerous projects in compliance with Good Automated Manufacturing Practice (GAMP). Under a project validation plan (PVP) that follows the V model described in the GAMP guide, experienced Yokogawa engineers deliver outcomes for the functional specification, design specification, implementation, factory testing, and site acceptance testing phases. The verification and test results, including the installation and operational qualifications (IQ /OQ), serve as objective indicators for determining if project requirements have been met.
Customer Challenge

Need for a more rigorous approach to ensure product quality
With pharmaceuticals, a rigorous scientific approach is needed to ensure product quality. The ICH quality guidelines call for a quality-by-design (QbD) approach in pharmaceutical development and manufacturing. Manufacturing facilities face the challenge of implementing innovative technologies such as process analytical technology (PAT) and are expected to introduce real-time release (RTR) testing.
Our Solutions
Yokogawa PAT Solution to Real-Time Release Testing (RTRT)
Yokogawa's approach to the development of process analytical technology (PAT) solutions relies on real-time monitoring of critical quality attributes (CQA) to achieve lean manufacturing. Online quality attributes can be directly monitored by means of near infrared (NIR) analysis. Another approach is the use of process modeling technology to monitor process health.
Resources
- Highlyadvanced biopharmaceutical plant uses CENTUM, VP Batch, Exaquantum and PRM.
- Total system integration for large-scale biotech production facility.
- Yokogawa provides CENTUM VP with Batch Recipe Management Package for new fine chemical plant.
- Yokogawa's CENTUM VP complies with the requirement of Good Automation Manufacturing Practices(GAMP).
- Uninterrupted Medical Oxygen Production with FAST/TOOLS Monitoring.
- Audits are performed periodically, requiring traceability and the keeping of historical records for all batch production activities.
- RotaMASS was chosen to work with weight measurements and/or differential pressure transmitters for extremely accurate measurements.
- The customer recognized the product's advantages: reliability, accuracy, guaranteed draining system, easily cleaned system, no moving parts, directly built into the pipelines, low pressure losses.
Kyowa Hakko Bio monitors vibration trends with Yokogawa's Sushi Sensors to prevent unexpected equipment failures.
By using eServ, sensor data and maintenance information are shared with everyone involved in manufacturing.
Bushu Pharmaceuticals, Ltd., a major contract manufacturing company, is working to eliminate human error to ensure the integrity of test results data, and to promote reliable inspections and paperless operations.
Establish thorough quality assurance with state-of-the-art facilities and technologies to foster the future of medical products.
When used to measure the dissolved oxygen in an aeration tank, sensors tend to quickly become contaminated and need frequent cleaning. A cost-effective solution to this problem has finally been found with the development of the Yokogawa DO402 Dissolved Oxygen Converter system.
With 462 million affected individuals, diabetes and associated conditions consume about 12% of global health expenditure. Current treatments lack focus on the root cause—the loss of pancreatic insulin-producing beta-cells. This Michigan research team developed a robust, intact ex vivo pancreatic islet bioassay capable of detecting diabetes-relevant endpoints including beta-cell proliferation, chemoprotection, and islet spatial morphometrics.
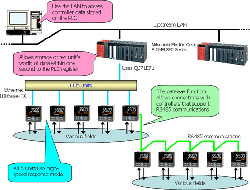
- For remote monitoring (of temperature, pressure, and flow volume), installing the SMARTDAC+ GM in the plant and the SMARTDAC+ GX in the office provides for a scalable, pc-free on-site data monitoring solution.
- You can centralize management of large quantities of data by automatically transferring acquired data to a FTP server.
High-capacity memory, network, and part 11 compliant paperless data recorders greatly increase work efficiency while eliminating data recording errors.
Prevent costly downtime and process equipment failures with real-time pressure monitoring. Yokogawa’s Sushi Sensor is the optimal choice for industries depending on bottled gas in essential procedures.
Avoid flashing issues while loading cryogenic liquids with safe, reliable, and accurate density fluid measurements utilizing Yokogawa’s industry-leading Coriolis flowmeter to eliminate measurement issues and increase the accuracy of inventory.
Cell stage categorized using FucciTime lapse imaging of Fucci-added Hela cells was conducted over 48 hrs at 1 hr intervals. Gating was performed based on the mean intensities of 488 nm and 561 nm for each cell. They were categorized into four stages, and the cell count for each was calculated.
Faster, Brighter, and More Versatile Confocal Scanner Unit
Applications: Colony Formation, Scratch Wound, Cytotoxicity, Neurite Outgrowth, Co-culture Analysis, Cell Tracking
Yokogawa’s distributed temperature sensors ensure comprehensive monitoring of thermal events in real-time, limiting facility damage by decreasing the risk of fire/duct explosions in exhaust air/fume ducts, allowing for early detection and quick response.
Eliminating the wire using wireless transmitter is the perfect solution for rotating equipment. It establishes data collection between the transmitter and the gateway by reliable communication even though the dryer was rotating.
SMARTDAC+ GX series records the clean room temperature, humidity, atmospheric pressure, door openings and closings, etc.
This introduces a system that uses Ethernet communications to acquire measured values, target values, and control output values from a controller installed on site into a PLC at high speed (ten units' worth within one second).
In semiconductor manufacturing processes, a deficient clean room environment can lead to defects and wasted resources, making a strictly controlled clean room indispensable. Maintaining the environment in the clean room requires control of air filters, HVAC systems, room temperature, humidity, airborne particles, and other factors.
In the plants of food and beverage manufacturers, there are times when monitoring and recording of production equipment is necessary inside clean rooms. This is an introduction to monitoring and recording in clean rooms using paperless recorders.
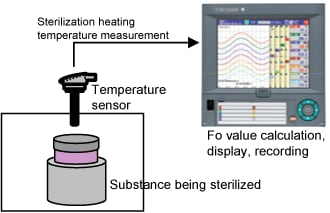
Performing control while changing the set point temperature moment by moment is called running a program pattern (or simply running a program). Sterilization and pasteurization require maintaining specific temperatures for specific durations.
A critical requirement in biopharmaceutical development is the integration and automation of process equipment and analytical instruments used in the laboratory. Bioprocess labs with multiple lab-scale bioreactors often execute cultivation experiments in parallel for research or process development purposes.
As part of a collaboration between Securecell (Zurich, SW) and Yokogawa Life Science (Tokyo, Japan), this application note demonstrates the effective use of the Lucullus® Process Information Management System (Lucullus®) to assist in the control of three Advanced Control Bioreactor Systems (BR1000) to study glucose utilization of CHO cells for optimal monoclonal antibody productivity.
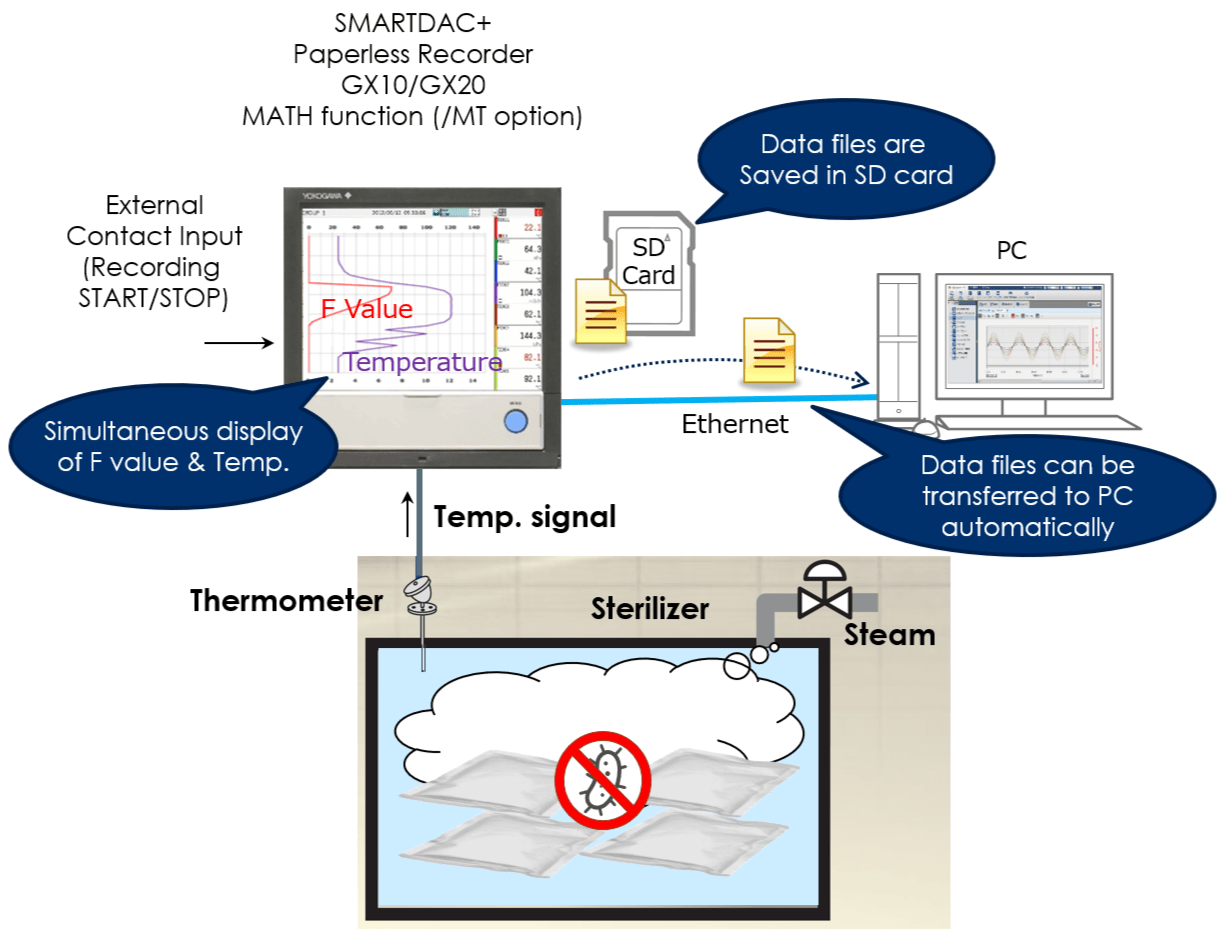
The paperless recorder DX series automatically calculate the Fo value from the heating temperature and are useful in managing the sterilization process by displaying and recording the value together with the heating temperature.
Clean-in-place (CIP) is the system designed for automatic cleaning and disinfecting in the food & beverage, pharmaceutical, and chemical industries. Tanks and piping are cleaned and sterilized with various cleaning solutions, fresh or hot water, or steam after manufacturing products is completed.
There are many points in the processing of edible fats and oils that benefit from the use of analytical measurements. Inductive conductivity, contacting conductivity, gas density, and pH can be utilized to increase the quality of the end product, as well as protecting expensive processes.
By using the Multibatch function (an option added with SMARTDAC+), you can efficiently record data from multiple devices onto a single SMARTDAC+.
Using the custom display function that comes standard with DXAdvanced means that you can combine the recorders, displays, and switches used in various kinds of equipment.
Using the batch name + lot number system, past measured data can be recalled for reference by batch name.
- Universal inputs provide support for thermocouple, RTD, voltage, and a variety of other input signals.
- Lineup of models for up to 450 inputs.
Allows multipoint monitoring and recording on a single unit. - Easily enables network-based data management.
file transfers, Web monitoring, and alarm e-mail
By using the computation function of the SMARTDAC+ series Paperless recorder GX/GP, computation option computes the "F-value," or sterilizing value for the sterilization process, so that the computation results can be recorded in the form of data.
Fluorescent ubiquitination-based cell cycle indicator (Fucci) is a set of fluorescent probes which enables the visualization of cell cycle progression in living cells.
The sterilization temperature prior to the filling process is monitored in the field or office. The temperature data is recorded in an external storage medium.
Blending plays a key role in industries such as food, healthcare and chemicals etc. Temperature and vacuum measurements are very important in minimizing the moisture content to ensure the quality of the final product. Strictly maintaining them throughout the process ensures the final product yield.
Measurement of pH in high purity water presents many difficulties. Yokogawa's pH instrument's design success comes from addressing the unique challenges of the application.
With industrial and economic development comes increasingly large and advanced power plants and factories. Nevertheless, we find many cases where the original cables, cable tunnels, and other components of the power infrastructure have languished under continuous operation.
In this Yokogawa RAP Best Practices eBook, you’ll discover how our wealth of knowledge about Integrated Safe Systems and Control of Work is available to help you and your teams design and implement a system that best suits your needs.
Yokogawa collaborates with scientists in the medical and pharmacology fields to identify best practices for cell painting and high-content screening, thereby enhancing image analysis and reproducibility.
This technical white paper will discuss Yokogawa's CENTUM VP DCS (Distributed Control System) product, hereafter referred to as "CENTUM VP", and the extent of its compliance with Part 11 of Title 21 of the Code of Federal Regulations, (21 CFR Part 11), the Electronic Records / Electronic Signatures Rule.
The worlds of process automation and production management have been converging for some time. What once used to be islands of automation and production management functionality connected through highly proprietary integration schemes that were costly to maintain have developed into integrated platforms that provide seamless data exchange between the world of automation and the plant floor, the functions of production and operations management, and integration with business level systems.
The world of process automation is governed by procedures. While we like to refer to the process industries as being largely "continuous", this could not be further from the truth. Process manufacturing is constantly in flux.
From engineering to installation, commissioning, operations, and maintenance, FOUNDATION fieldbus offer significant cost reductions of 30 percent or more versus conventional analog systems. Many of these cost reductions come from the advanced functions that fieldbus offers versus analog technology.
The whitepaper discusses the importance of safety culture in achieving a smarter and safer working environment. It highlights statistics on work-related accidents and diseases, the cost of poor safety culture, and the need for a true safety culture achieved through company leadership.
This document describes the operation and data flow of the Yokogawa Print Wave software using the DX-P Reporter. It will provide a detailed explanation of the Advanced Alarm Reporter functions. The functions described in this paper were first released in Print Wave version 5.5.
Discovering your Baseline with OT Security Risk Assessment
On-site digital champions can drive your operation towards data integration and a more aligned way of working.
Smart devices, like Yokogawa’s line of Total Insight transmitters and flowmeters contribute to Digital Transformation during operations and throughout the lifecycle of the instrument.
The first stage in creating next-generation production control system that achieves innovative plant operation is providing "Visualized operation". "Visualized operation" for the customer means that he can reliably access and visualize plant data regardless of plant location and time.
Lonza's Riverside plant manufactures bulk active ingredients for the pharmaceutical industry. A new, fully automated multipurpose reactor train was installed in early 1996. The train includes ten 500 to 1500 gallon vessels used for reaction, distillation, phase separation, and crystallization; two centrifuges for isolating finished products; and two dryers.
Manufacturers that are digitalizing lab information management can improve quality and streamline operations. Read the complete article inside.
The critical steps in achieving advanced process control include building an accurate digital twin of a bioreactor, validating it, and establishing communication between the bioreactor and its digital twin. Several of these steps are already mature and Yokogawa is making rapid progress toward realizing ‘smart’ bioreactors for smart manufacturing.
Exploring the technologies needed to achieve automatic control of cell culture processes.
Unlocking the Keys to a Sustainable Pharma Supply Chain
Yokogawa Insilico Biotechnology released its Insilico Digital Twin Factory, which can help drugmakers increase productivity and bring down manufacturing costs and time to market by potentially replacing up to 50% of the experiments needed during the process development, characterization and scale-up of biopharmaceuticals.
Kind of. Calibration itself will not extend the life of a sensor, however, a sensor that is not calibrated properly can cause unreliable measurements - that are often misdiagnosed leading to unnecessary replacements.
Optimizing the maintenance cycle is not always straightforward. In some cases, cleaning once a week is sufficient and other processes may require every 8 hours.
The lifetime of a pH sensor has a significant impact on the overall annual costs of a pH measuring loop. Optimizing four key factors will decrease these costs and optimize process control and overall plant efficiency.
Learning these four lessons will help you improve your engineering skills and most importantly extend the life of your pH sensors.
Downloads
Brochures
- CellVoyager CQ1 Benchtop High-Content Analysis System (2.9 MB)
- Inside a Hidden World (6.1 MB)
- Yokogawa in the Pharmaceutical Industry (7.7 MB)
- Yokogawa Corporation of America Corporate Overview - Products & Solutions (3.5 MB)
- CSU-W1 Confocal Scanner Unit (2.9 MB)
- CSU-X1 Confocal Scanner Unit (2.4 MB)
- Yokogawa CSU-W1 SoRa Confocal Scanner Unit (525 KB)
- Hygienic and Sanitary Solutions for Pressure and Level Applications EJA565E/EJA564E (3.8 MB)
Instruction Manuals
Technical Information
Certificates
Videos
In this video you will learn about flow imaging microscopy, and how the FlowCam works to deliver digital images of subvisible particles.
This is a 2-minute promotional movie for OpreX LIMS. It will give you a general idea of what it is.
Fast, gentle, and clear - live-cell imaging. Yokogawa's unique scanning method minimizes damage to living cells and organisms and even can capture faint/fast life phenomena.
More than 2,500+ units scanning units sold worldwide. This fast, reliable, and accurate technology has been leading cutting-edge research and supporting researchers around the world for more than two decades.
More information: https://www.yokogawa.com/us/solutions/products-platforms/life-science/spinning-disk-confocal/
#confocal #microlens #microscope #CSU #Yokogawa #livecell
Yokogawa's CQ1 open platform integrates seamlessly with Advanced Solutions BioAssemblyBot® 400. With laboratory automation becoming a standard in research, Yokogawa's high content confocal system's ability to work with robots like Advanced Solutions' BioAssemblyBot® 400 is essential to advancing laboratory automation.
A demonstration of the FlowCam 8000 and how it works for marine and freshwater research and monitoring
A demonstration of the FlowCam 8000 for pharma and biopharma applications
A demonstration of the FlowCam 8000 for industrial applications
Open up control. Open Process Automation (OPA) opens the door to expanded choices, leverage, and customization.
Create a scalable, sustainable architecture. Remove the restraints of proprietary systems. OPA enables you to leverage all available technologies to open up solutions. Explore the possibilities today.
As a gateway to further expand Yokogawa's messaging as a One-stop Solution Business, Yokogawa has partnered up with INCIT, to introduce the S.I.R.I. framework.
This video describes the process of connecting the Yokogawa Magnetic Flow Meter ADMAG TI - AXG with EtherNet/IP to a webserver
This video explains how to open .eds files in Studio 5000. The Yokogawa Magnetic Flow Meter ADMAG TI - AXG with EtherNet/IP is used as an example.
This video covers the navigation and basic structure of the Yokogawa Magnetic Flow Meter ADMAG TI - AXG with EtherNet/IP webserver.
> Video: How to Connect to Webserver
This video shows steps to bring the Studio 5000 Data Points into the FactoryTalk View Studio. The Yokogawa Magnetic Flow Meter ADMAG IT - AXG with EtherNet/IP is shown as an example.
Welcome to a transformative journey in the pharmaceutical industry!
Today, we're diving into how we, Yokogawa are reshaping the pharmaceutical landscape. Our focus? Innovation and digitalization. This isn't just about technology; it's about ensuring a stable, reliable supply of medicines to those who need them most.
We're tackling key industry challenges: enhancing flexibility and agility, reducing time to market, and boosting process efficiency.
How? By harnessing the power of data. Join us as we explore how Yokogawa's cutting-edge solutions are making "right-first-time" and "zero-waste" drug manufacturing a reality. From on-premise to cloud-based solutions, we're bringing smarter, more secure, and more cost-effective production to life. Let's uncover the future of pharmaceuticals, powered by Yokogawa!
For more information: https://www.yokogawa.com/us/industries/pharmaceutical/
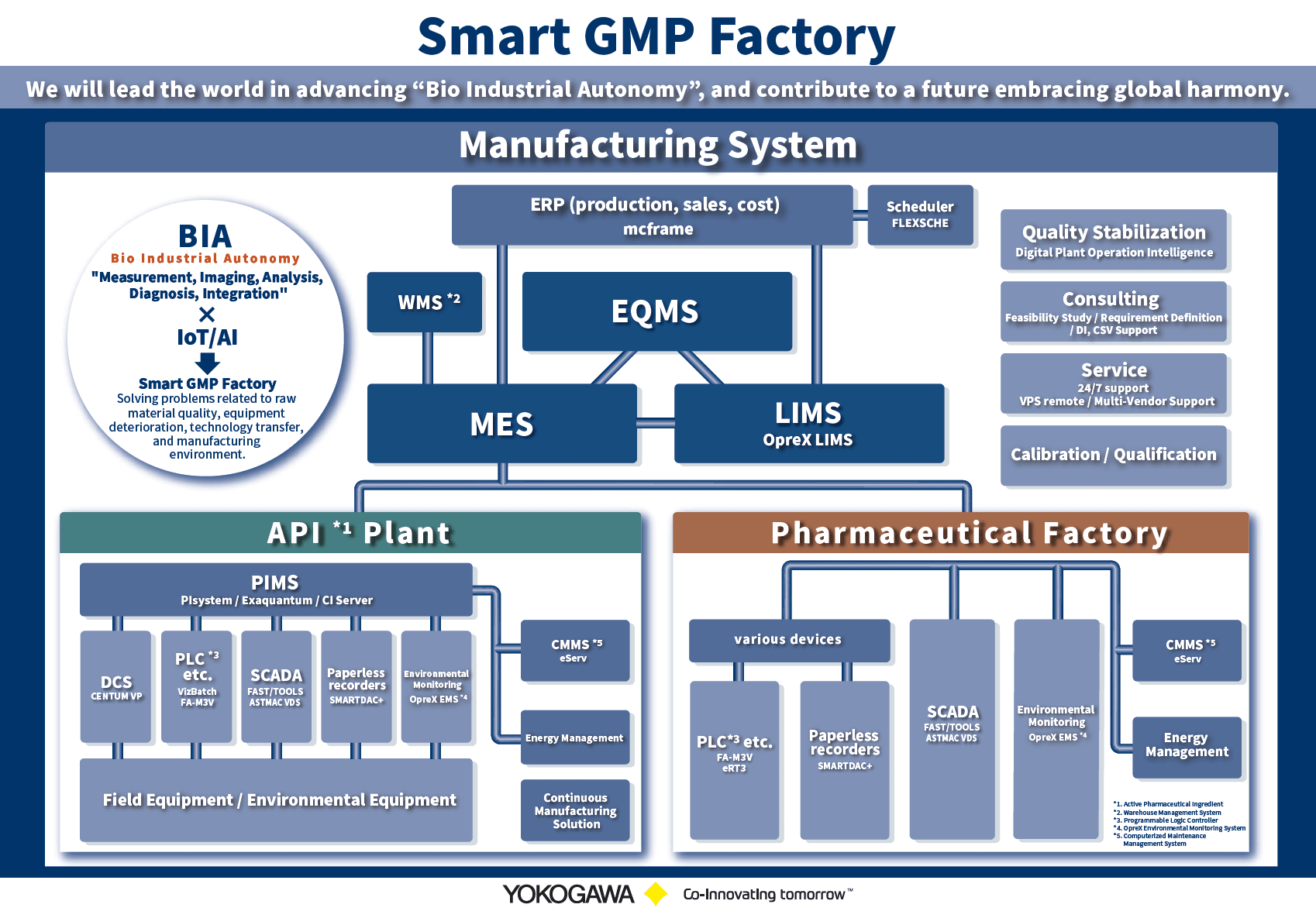
Yokogawa leverages our extensive knowledge and experience in IT, operation and control technology to build an advanced improvement cycle that is useful to all management levels, and eventually aims to achieve Pharma 4.0 together with our customers.
Physiologically relevant 3D cell models are being adopted for disease modeling, drug discovery and preclinical research due to their functional and architectural similarity to their tissue/sample of origin, especially for oncology research. Multifunctional profiling and assays using 3D cell models such as tumoroids tend to be manual and tedious. Further, high-content imaging of biomarkers in 3D cell models can be difficult.
In this two-part webinar present to you streamlined technologies which can bring consistent timesaving, ease-of-use, and high-quality data to your 3D cell-based workflows:
(A) The Pu·MA System is a microfluidics-based benchtop automated device for performing “hands-off” 3D cell-based assays. In this webinar, application scientist Dr. Katya Nikolov will present data from optimized assays using tumoroids followed by Yokogawa’s high-content imaging systems for biomarker detection.
(B) Yokogawa’s high-content imaging systems such as CellVoyager CQ1 provide superior confocal imaging using the Nipkow Spinning Disk Confocal Technology. Here, application scientist, Dan Collins will present details of the high-content imaging capabilities, easy to use and intuitive image acquisition software, especially for increasing productivity and a streamlined workflow.
Learn How:
- The open platform, Pu·MA System can be used to automate your 3D cell-based assays
- To perform automated IF staining for biomarkers using tumoroid models without perturbing your precious samples
- Image acquisition from 3D cell models using Yokogawa’s high-content imaging platforms
- Image analysis from cells, complex spheroids, colonies, or tissues using the CellPathfinder high content analysis software
In this webinar, Professor Jonny Sexton discusses a pipeline, developed in the Sexton lab, for the quantitative high-throughput image-based screening of SARS-CoV-2 infection to identify potential antiviral mechanisms and allow selection of appropriate drug combinations to treat COVID-19. This webinar presents evidence that morphological profiling can robustly identify new potential therapeutics against SARS-CoV-2 infection as well as drugs that potentially worsen COVID-19 outcomes.
Physiologically relevant 3D cell models are essential for drug discovery and preclinical research due to their functional and architectural similarity to solid tumors. One of the challenges faced by researchers is that many of the assays using these precious samples tend to be manual and tedious.
Using proprietary microfluidics technology, Protein Fluidics has created the Pu·MA System for automated complex 3D cell-based assays. In this webinar, application scientist Dr. Katya Nikolov will present her work on combining this novel automation technology with Yokogawa’s high-content imaging systems for biomarker detection in 3D cell models. Nikolov will demonstrate the utility of an automated immunofluorescence staining workflow followed by confocal imaging within the Pu·MA System flowchips. This automated workflow enables quantitative assessment of biomarkers which provides valuable data for further understanding disease mechanisms, preclinical drug efficacy studies, and in personalized medicine.
This webinar will explore:
- The Pu·MA System and novel technology for automated 3D cell-based assays
- How to perform automated immunofluorescence staining for biomarkers with a “hands-off” assay workflow
- How to visualize biomarkers after the assay with high-content imaging within the flowchip
This webinar highlights Yokogawa’s High Content Solutions, the benchtop confocal CellVoyager CQ1, and CellVoyager CV8000. Utilizing Yokogawa’s dual-wide microlens spinning disk confocal technology, these automated HCA systems provide remarkable image quality while increasing your output. This frees up time to complete other research activities. Also, recent additions to the CSU-W1 confocal upgrade is discussed. The SoRa, a super-resolution solution, and the Uniformizer, an image flattening device. Both of which can be added to the lightpath of your CSU-W1-enhanced microscope.
Agenda:
Introduction to Yokogawa
SoRa for CSU-W1 super-resolution with confocal
Two high content instruments from Yokogawa: The CQ1 and the CV8000
Presenter:
Dan J. Collins, Applications Scientist, Yokogawa Life Science
In the last few decades, the pharmaceutical industry has transformed people’s lives. However, the development of new drugs is becoming increasingly difficult and a paradigm shift in the drug discovery workflow is required to reduce attrition and transform conventional drug screening assays into translatable analytical techniques for the analysis of drugs in complex environments, both in-vitro and ex-vivo. The ability to visualize unlabelled compounds inside the cell at physiological dosages can offer valuable insight into the compound behavior both on and off-target.
SiLC-MS is a semi-automated methodology that allows the collection of intracellular contents using a modified CQ1 imaging system developed by Yokowaga. The instrument is equipped with a confocal microscope that allows bright field imaging as well as fluorescence imaging with 4 lasers (405, 488, 561, and 640 nm). Sampling is performed using the tips developed by Professor Masujima (1-4).
In this study, we show the applicability of the SiLC-MS technology to drug discovery, as it is crucial to identify compound and its metabolites when incubated in a mammalian cell at a therapeutic dose. We report on the validation studies performed using the SiLC-MS platform, in these validation studies we assess the ability to distinguish different cell types based on their metabolomic fingerprint, furthermore, we have also evaluated if this assay was sensitive enough to detect drugs intracellularly.
Presenter: Carla Newman, Scientific Leader (Celluar Imaging and Dynamics), GSK
Generating translatable high-content imaging data from physiologically-relevant cell models, including 2D and 3D structures, is extremely valuable for drug discovery and pre-clinical research. In this webinar, James Evans, CEO of PhenoVista Biosciences presents case studies on how Yokogawa’s Benchtop CQ1 Confocal System can improve throughput and standardize processes for complex 3D cell-based phenotypic assays.
Key learning objectives:
- Strategies for designing and implementing high-content screening assays
- Approaches for deciding between 2D and 3D model systems
Human pluripotent stem cells are proven efficient models for drug screening campaigns. They provide access to unlimited starting material amenable to high throughput small molecule compound screening. Due to their capacity to generate mode complex cellular models, they also offer the potential to perform high content screening in tissue-specific organoids for further human target validation.
Dr. Alejandro Hidalgo-Gonzalez at MCRI(Murdoch Children's Research Institute) in Australia is a user of our HCA system CellVoyager CV8000 and he has established a pipeline for assay development and automated unbiased phenotypic drug screening using human pluripotent stem cell-derived cells and organoids. This is a recording of his presentation at an educational webinar organized by A*STAR in Singapore.
3D imaging experts from Yokogawa and Insphero have come together to provide helpful tips and tricks on acquiring the best 3D spheroid and organoid imaging. This webinar focuses on sample preparation, imaging, and analysis for both fixed and live cells in High Content Screening assays. The experts also discuss automated tools that can help researchers understand the large volume of data in these High Content Imaging Analysis Systems.
Discover how to achieve industrial autonomy step by step through one of the 75+ inspiring presentations.
Integration of process analytical technologies (PAT) with bioreactor control systems is critical for automation of glucose feed management in mammalian cell culture. The Yokogawa BR1000 Advanced Control Bioreactor System comprehensively integrates in-line sensing, intelligent model predictive control software, CHO cell model development capabilities, and automated reagent delivery.
During this webinar and user experience event, attendees will:
- See how customized cell models can be created specifically for any cell expression system
- Get a close-up look at the BR1000's user-friendly design
- Learn what sets the BR1000 apart from typical bioreactors
- View a video demonstration of the instrument's set-up, calibration, prep, loading, and start-up
To demonstrate the power of data collection and integration for bioprocess control, we have applied the Lucullus® Process Information Management System (Securecell) together with the BR1000 Advanced Control Bioreactor System (Yokogawa). In this joint webinar, it will be shown how Lucullus plays a key role in the automated control of three BR1000 bioreactors during a glucose-shift study to optimize monoclonal antibody productivity in CHO cells.
See how streamlined workflows and data management across laboratory devices, users and software systems can lead to new options and advance biologic development activities in this example.
Visualizing the complex spatiotemporal dynamics of human stem cells as they proliferate and make cell fate decisions is key to improving our understanding of how to robustly engineer differentiated tissues for therapeutic applications.
In this webinar, Dr. Rafael Carazo Salas will describe multicolor, multiday high-content microscopy pipelines that his group has recently developed to visualize the dynamical cell fate changes of human Pluripotent Stem Cells (hPSCs).
Key Topics:
- Visualizing how human Pluripotent Stem Cells (hPSCs) proliferate and undergo early differentiation in vitro, by high content microscopy
- Learning about experimental and computational pipelines that enable monitoring single-cell fate dynamics
- Learning about novel “live” reporters of hPSC cell fate
Speaker
Rafael Carazo Salas, PhD
Professor, School of Cellular and Molecular Medicine
University of Bristol
Image-based phenotypic screening relies on the extraction of multivariate information from cells cultured in a large number of screened conditions. In this webinar, we explored the application of complex and biologically relevant model systems for drug screening, such as small intestinal organoids.
Key topics include:
- Learn how to upscale, streamline, and automate intestinal organoid handling
- Learn how to image in complex three-dimensional (3D) model systems and how to approach large imaging datasets
- Understand the basics of multivariate analysis on image-inferred features
In this webinar, we will:
- Share best practices for monitoring, recording, and transferring data
- Challenges in data acquisition and how Yokogawa can help
- Demonstrate GA10 Data Logging Software capabilities
In this webinar, we will:
- Introduce Yokogawa AI, including Sushi Sensors
- Demonstrate GA10 AI Dashboard and simple setup
- Discuss applications and real-world examples
In this webinar, industry-leading experts will describe how OPAF is selecting standards that will achieve the vision of truly open systems, the status of its efforts, and what this exciting change to the process automation world means to you.
Sample systems are a crucial component of and have a significant impact on the performance of a process gas chromatograph analyzer. When working on sample systems, you must have a constant awareness of time delays. This fundamentals webinar will take the mystery out of lag times.
This workshop provided by industry partners Yokogawa and ThreatConnect will show you a way to strategically manage cyber risk within an industrial environment. Learn how to transition from the “break/fix” model of industrial cybersecurity to a business risk management strategy through cyber risk quantification, return on investment (ROI) for industrial cybersecurity solutions calculations, and cyber risk identification.
News
-
News Brief Apr 25, 2024 Yokogawa Helps to Revolutionize the Field of Single-Cell Lipidomics
-
Press Release Jan 31, 2024 Yokogawa Introduces CellVoyager High-Content Analysis System CQ3000
- For greater efficiency in drug discovery and regenerative medicine R&D, and the swift commercialization of new drugs -
-
Press Release Jun 21, 2023 Yokogawa to Release OpreX Informatics Manager, Enabling Integrated Management of Experimental Data and Research Resources in the Cloud
-
Press Release Feb 20, 2023 Yokogawa Enters into Partnership with Radial Software Group to Provide AI-powered Viewport Software Worldwide
- Giving customers a single view of all their technical data -
-
Press Release Apr 1, 2022 Notice of Commencement of Business of Pharmira Co., Ltd., a Joint Venture for Contract Development and Manufacturing of Active Pharmaceutical Ingredients and Intermediates
Looking for more information on our people, technology and solutions?
Contact Us